Strategic Tools in Regenerative and Translational Dentistry
Abstract
:1. Introduction
2. Stem Cells from Oral Tissues
3. In vitro Manipulation of ODSCs: the Good the Bad and the Ugly
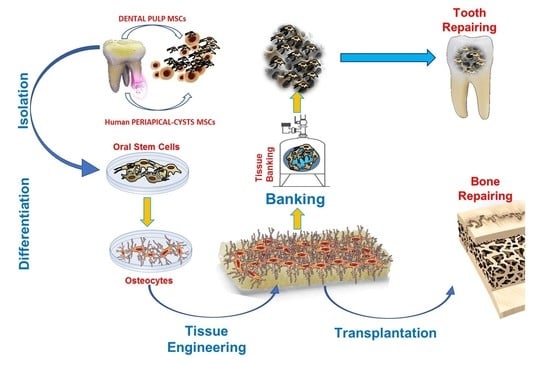
3.1. ODSCs Applications: from Bench to Dental-Chair Side
3.2. Scaffolds-Supported Tissue Engineering Protocols
3.3. Alternative Approaches in Regenerative/Reparative Dentistry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Lo Giudice, R.; Matarese, G.; Isola, G.; Cicciù, M.; Terranova, A.; Palaia, G.; Romeo, U. Evaluation of magnification systems in restorative dentistry. An in-vitro study. Dent. Cadmos 2015, 83, 296–305. [Google Scholar] [CrossRef]
- Isola, G.; Cicciu, M.; Fiorillo, L.; Matarese, G. Association between odontoma and impacted teeth. J. Craniofac. Surg. 2017, 28, 755–758. [Google Scholar] [CrossRef]
- Tatullo, M. About stem cell research in dentistry: Many doubts and too many pitfalls still affect the regenerative dentistry. Int. J. Med. Sci. 2018, 15, 1616–1618. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Paduano, F. The regenerative medicine in oral and maxillofacial surgery: The most important innovations in the clinical application of mesenchymal stem cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reichert, T.E. Dental stem cells in tooth regeneration and repair in the future. Expert Opin. Biol. Ther. 2018, 18, 187–196. [Google Scholar] [CrossRef]
- Ghiroldi, A.; Piccoli, M.; Cirillo, F.; Monasky, M.M.; Ciconte, G.; Pappone, C.; Anastasia, L. Cell-based therapies for cardiac regeneration: A comprehensive review of past and ongoing strategies. Int. J. Mol. Sci. 2018, 19, 3194. [Google Scholar] [CrossRef]
- Rozier, P.; Maria, A.; Goulabchand, R.; Jorgensen, C.; Guilpain, P.; Noel, D. Mesenchymal stem cells in systemic sclerosis: Allogenic or autologous approaches for therapeutic use? Front. Immunol. 2018, 9, 2938. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M. Science is not a Social Opinion. Dent. J. 2019, 7, 34. [Google Scholar] [CrossRef]
- Mantesso, A.; Sharpe, P. Dental stem cells for tooth regeneration and repair. Expert Opin. Biol. Ther. 2009, 9, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Beyer Nardi, N.; da Silva Meirelles, L. Mesenchymal stem cells: Isolation, in vitro expansion and characterization. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 249–282. [Google Scholar]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral infection by staphylococcus aureus in patients affected by white sponge nevus: A description of two cases occurred in the same family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can autofluorescence guide surgeons in the treatment of medication-related osteonecrosis of the jaw? A prospective feasibility study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: Case report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef]
- Figliuzzi, M.M.; Giudice, A.; Pileggi, S.; Pacifico, D.; Marrelli, M.; Tatullo, M.; Fortunato, L. Implant-prosthetic rehabilitation in bilateral agenesis of maxillary lateral incisors with a mini split crest. Case Rep. Dent. 2016, 2016, 3591321. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Shakesheff, K.M.; White, L.J. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2015, 9, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Falisi, G.; Amantea, M.; Rastelli, C.; Paduano, F.; Marrelli, M. Dental pulp stem cells and human periapical cyst mesenchymal stem cells in bone tissue regeneration: Comparison of basal and osteogenic differentiated gene expression of a newly discovered mesenchymal stem cell lineage. J. Biol. Regul. Homeost Agents 2015, 29, 713–718. [Google Scholar]
- Marrelli, M.; Pujia, A.; Palmieri, F.; Gatto, R.; Falisi, G.; Gargari, M.; Caruso, S.; Apicella, D.; Rastelli, C.; Nardi, G.M.; et al. Innovative approach for the in vitro research on biomedical scaffolds designed and customized with CAD-CAM technology. Int. J. Immunopathol. Pharmacol. 2016, 29, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Iohara, K.; Zheng, L.; Ito, M.; Tomokiyo, A.; Matsushita, K.; Nakashima, M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 2006, 24, 2493–2503. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. Shed: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Morsczeck, C.; Gotz, W.; Schierholz, J.; Zeilhofer, F.; Kuhn, U.; Mohl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Yi, T.; Lee, S.; Choi, N.; Shin, H.S.; Kim, J.; Lim, J.Y. Single cell clones purified from human parotid glands display features of multipotent epitheliomesenchymal stem cells. Sci. Rep. 2016, 6, 36303. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003, 9 (Suppl. 1), S45–S64. [Google Scholar] [CrossRef]
- Tomar, G.B.; Srivastava, R.K.; Gupta, N.; Barhanpurkar, A.P.; Pote, S.T.; Jhaveri, H.M.; Mishra, G.C.; Wani, M.R. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem. Biophys. Res. Commun. 2010, 393, 377–383. [Google Scholar] [CrossRef]
- Yang, B.; Qiu, Y.; Zhou, N.; Ouyang, H.; Ding, J.; Cheng, B.; Sun, J. Application of stem cells in oral disease therapy: Progresses and perspectives. Front. Physiol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Gaggi, G.; Izzicupo, P.; Di Credico, A.; Sancilio, S.; Di Baldassarre, A.; Ghinassi, B. Spare parts from discarded materials: Fetal annexes in regenerative medicine. Int. J. Mol. Sci. 2019, 20, 1573. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Muralitharan, R.; Gargett, C.E. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin. Biol. Ther. 2013, 13, 1387–1400. [Google Scholar] [CrossRef]
- Marrelli, M.; Paduano, F.; Tatullo, M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J. Dent. Res. 2015, 94, 843–852. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Palmieri, F.; Tatullo, M. Cd146 expression influences periapical cyst mesenchymal stem cell properties. Stem Cell Rev. 2016, 12, 592–603. [Google Scholar] [CrossRef]
- Tatullo, M.; Codispoti, B.; Pacifici, A.; Palmieri, F.; Marrelli, M.; Pacifici, L.; Paduano, F. Potential use of human periapical cyst-mesenchymal stem cells (hPCy-MSCs) as a novel stem cell source for regenerative medicine applications. Front. Cell Dev. Biol. 2017, 5, 103. [Google Scholar] [CrossRef]
- Ikeda, E.; Yagi, K.; Kojima, M.; Yagyuu, T.; Ohshima, A.; Sobajima, S.; Tadokoro, M.; Katsube, Y.; Isoda, K.; Kondoh, M.; et al. Multipotent cells from the human third molar: Feasibility of cell-based therapy for liver disease. Differentiation 2008, 76, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Chen, M.F.; Liao, C.H.; Pang, C.Y.; Lin, P.Y. A simple and efficient method for generating nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy 2009, 11, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Sun, H.H.; Lu, H.; Yu, Q. Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012, 33, 6320–6344. [Google Scholar] [CrossRef]
- Racz, G.Z.; Kadar, K.; Foldes, A.; Kallo, K.; Perczel-Kovach, K.; Keremi, B.; Nagy, A.; Varga, G. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J. Physiol. Pharmacol. 2014, 65, 327–339. [Google Scholar]
- Suchanek, J.; Visek, B.; Soukup, T.; El-Din Mohamed, S.K.; Ivancakova, R.; Mokry, J.; Aboul-Ezz, E.H.; Omran, A. Stem cells from human exfoliated deciduous teeth-isolation, long term cultivation and phenotypical analysis. Acta Medica (Hradec Kralove) 2010, 53, 93–99. [Google Scholar] [CrossRef]
- Kerkis, I.; Caplan, A.I. Stem cells in dental pulp of deciduous teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- Patil, R.; Kumar, B.M.; Lee, W.J.; Jeon, R.H.; Jang, S.J.; Lee, Y.M.; Park, B.W.; Byun, J.H.; Ahn, C.S.; Kim, J.W.; et al. Multilineage potential and proteomic profiling of human dental stem cells derived from a single donor. Exp. Cell Res. 2014, 320, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liu, Y.; Lu, W.; Yang, F.; Yu, J.; Wang, X.; Ma, Q.; Yang, Z.; Wen, L.; Xuan, K. Periodontal tissue engineering with stem cells from the periodontal ligament of human retained deciduous teeth. J. Periodontal Res. 2013, 48, 105–116. [Google Scholar] [CrossRef]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nor, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- Stem cells from wisdom teeth transformed into corneal cells. Dent. Today 2015, 34, 54.
- Thirumala, S.; Goebel, W.S.; Woods, E.J. Manufacturing and banking of mesenchymal stem cells. Expert Opin. Biol. Ther. 2013, 13, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Dricu, A. Recent challenges with stem cell banking. Expert Opin. Biol. Ther. 2018, 18, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Paduano, F.; Tatullo, M. Cells isolated from human periapical cysts express mesenchymal stem cell-like properties. Int. J. Biol. Sci. 2013, 9, 1070–1078. [Google Scholar] [CrossRef]
- Marrelli, M.; Gentile, S.; Palmieri, F.; Paduano, F.; Tatullo, M. Correlation between surgeon’s experience, surgery complexity and the alteration of stress related physiological parameters. PLoS ONE 2014, 9, e112444. [Google Scholar] [CrossRef] [PubMed]
- Kawano, E.; Toriumi, T.; Iguchi, S.; Suzuki, D.; Sato, S.; Honda, M. Induction of neural crest cells from human dental pulp-derived induced pluripotent stem cells. Biomed. Res. 2017, 38, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Gao, Z.; Xu, J.; Zhu, Z.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Decellularized swine dental pulp as a bioscaffold for pulp regeneration. BioMed Res. Int. 2017, 2017, 9342714. [Google Scholar] [CrossRef]
- Tamaoki, N.; Takahashi, K.; Tanaka, T.; Ichisaka, T.; Aoki, H.; Takeda-Kawaguchi, T.; Iida, K.; Kunisada, T.; Shibata, T.; Yamanaka, S.; et al. Dental pulp cells for induced pluripotent stem cell banking. J. Dent. Res. 2010, 89, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle extracellular matrix scaffold is a multipotent environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental pulp stem cell mechanoresponsiveness: Effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
- Simu, M.R.; Pall, E.; Radu, T.; Miclaus, M.; Culic, B.; Mesaros, A.S.; Muntean, A.; Filip, G.A. Development of a novel biomaterial with an important osteoinductive capacity for hard tissue engineering. Tissue Cell 2018, 52, 101–107. [Google Scholar] [CrossRef]
- Bottino, M.C.; Pankajakshan, D.; Nor, J.E. Advanced scaffolds for dental pulp and periodontal regeneration. Dent. Clin. N. Am. 2017, 61, 689–711. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Albuquerque, M.T.; Evans, J.D.; Kamocka, M.M.; Gregory, R.L.; Bottino, M.C. Triple antibiotic polymer nanofibers for intracanal drug delivery: Effects on dual species biofilm and cell function. J. Endod. 2016, 42, 1490–1495. [Google Scholar] [CrossRef]
- Daniels, A.U.; Andriano, K.P.; Smutz, W.P.; Chang, M.K.; Heller, J. Evaluation of absorbable poly(ortho esters) for use in surgical implants. J. Appl. Biomater. 1994, 5, 51–64. [Google Scholar] [CrossRef]
- DuRaine, G.D.; Brown, W.E.; Hu, J.C.; Athanasiou, K.A. Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Ann. Biomed. Eng. 2015, 43, 543–554. [Google Scholar] [CrossRef]
- Shimomura, K.; Ando, W.; Fujie, H.; Hart, D.A.; Yoshikawa, H.; Nakamura, N. Scaffold-free tissue engineering for injured joint surface restoration. J. Exp. Orthop. 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paduano, F.; Marrelli, M.; Amantea, M.; Rengo, C.; Rengo, S.; Goldberg, M.; Spagnuolo, G.; Tatullo, M. Adipose tissue as a strategic source of mesenchymal stem cells in bone regeneration: A topical review on the most promising craniomaxillofacial applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.I.; Ohba, S.; Chikazu, D. Bone regeneration by human dental pulp stem cells using a helioxanthin derivative and cell-sheet technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatullo, M.; Simone, G.M.; Tarullo, F.; Irlandese, G.; Vito, D.; Marrelli, M.; Santacroce, L.; Cocco, T.; Ballini, A.; Scacco, S. Antioxidant and antitumor activity of a bioactive polyphenolic fraction isolated from the brewing process. Sci. Rep. 2016, 6, 36042. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; El-Husseny, M.W.A.; Abushouk, A.I.; Salem, A.M.A.; Mamdouh, M.; Abdel-Daim, M.M. Effects of antioxidant supplements on the survival and differentiation of stem cells. Oxid. Med. Cell. Longev. 2017, 2017, 5032102. [Google Scholar] [CrossRef]
- Kleineidam, B.; Nokhbehsaim, M.; Deschner, J.; Wahl, G. Effect of cold plasma on periodontal wound healing-an in vitro study. Clin. Oral Investig. 2019, 23, 1941–1950. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells Int. 2018, 2018, 3057624. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Diomede, F.; Ballerini, P.; Paolantonio, M.; Marchisio, M.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. The secretome of periodontal ligament stem cells from MS patients protects against EAE. Sci. Rep. 2016, 6, 38743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zomer, A.; Vendrig, T.; Hopmans, E.S.; van Eijndhoven, M.; Middeldorp, J.M.; Pegtel, D.M. Exosomes: Fit to deliver small RNA. Commun. Integr. Biol. 2010, 3, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, B.; Marrelli, M.; Paduano, F.; Tatullo, M. Nanometric bio-banked MSC-derived exosome (nanobiome) as a novel approach to regenerative medicine. J. Clin. Med. 2018, 7, 357. [Google Scholar] [CrossRef]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In vitro long-term expansion and high osteogenic potential of periodontal ligament stem cells: More than a mirage. Cell Transplant. 2019, 28, 129–139. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. https://doi.org/10.3390/ijms20081879
Tatullo M, Codispoti B, Paduano F, Nuzzolese M, Makeeva I. Strategic Tools in Regenerative and Translational Dentistry. International Journal of Molecular Sciences. 2019; 20(8):1879. https://doi.org/10.3390/ijms20081879
Chicago/Turabian StyleTatullo, Marco, Bruna Codispoti, Francesco Paduano, Manuel Nuzzolese, and Irina Makeeva. 2019. "Strategic Tools in Regenerative and Translational Dentistry" International Journal of Molecular Sciences 20, no. 8: 1879. https://doi.org/10.3390/ijms20081879
APA StyleTatullo, M., Codispoti, B., Paduano, F., Nuzzolese, M., & Makeeva, I. (2019). Strategic Tools in Regenerative and Translational Dentistry. International Journal of Molecular Sciences, 20(8), 1879. https://doi.org/10.3390/ijms20081879