Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients
Abstract
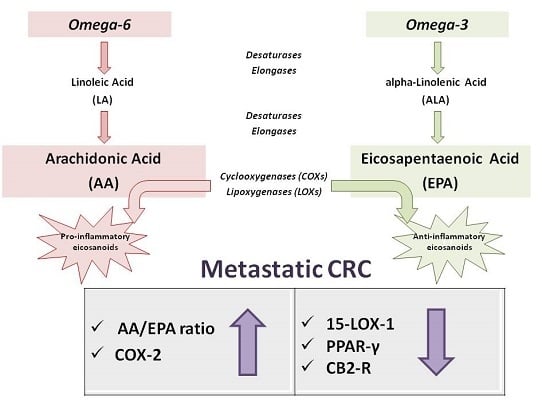
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. AA/EPA Ratio Assay
4.3. Gene Expression Analysis
4.4. Western Blotting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesic, M.; Greten, F.R. Inflammation and cancer: Tissue regeneration gone awry. Curr. Opin. Cell Biol. 2016, 43, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Rao, C.V. The role of inflammation in colon cancer. Adv. Exp. Med. Biol. 2014, 816, 25–52. [Google Scholar]
- Francuz, T.; Czajka-Francuz, P.; Cison-Jurek, S.; Wojnar, J. the role of inflammation in colon cancer pathogenesis. Postepy Hig. Med. Dosw (Online) 2016, 70, 360–366. [Google Scholar] [CrossRef]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, M.; Caruso, M.G.; Tutino, V.; De Nunzio, V.; Gigante, I.; De Leonardis, G.; Veronese, N.; Rotolo, O.; Reddavide, R.; Stasi, E.; et al. Nutrition and lipidomic profile in colorectal cancers. Acta Biomed. 2018, 89, 87–96. [Google Scholar] [PubMed]
- Wang, S.; Xie, J.; Li, H.; Yang, K. Differences of polyunsaturated fatty acid in patients with colorectal cancer and healthy people. J. Cancer Res. Ther. 2015, 11, 459–463. [Google Scholar]
- Coviello, G.; Tutino, V.; Notarnicola, M.; Caruso, M.G. Erythrocyte membrane fatty acids profile in colorectal cancer patients: A preliminary study. Anticancer. Res. 2014, 34, 4775–4779. [Google Scholar]
- Kim, H.Y.; Lee, K.M.; Kim, S.H.; Kwon, Y.J.; Chun, Y.J.; Choi, H.K. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget 2016, 7, 67111–67128. [Google Scholar] [CrossRef] [Green Version]
- Long, J.; Zhang, C.J.; Zhu, N.; Du, K.; Yin, Y.F.; Tan, X.; Liao, D.F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in lipidomics for cancer biomarkers discovery. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Notarnicola, M.; Lorusso, D.; Tutino, V.; De Nunzio, V.; De Leonardis, G.; Marangelli, G.; Guerra, V.; Veronese, N.; Caruso, M.G.; Giannelli, G. Differential tissue fatty acids profiling between colorectal cancer patients with and without synchronous metastasis. Int. J. Mol. Sci. 2018, 19, E962. [Google Scholar] [CrossRef]
- Kitagawa, M.; Haji, S.; Amagai, T. Elevated serum aa/epa ratio as a predictor of skeletal muscle depletion in cachexic patients with advanced gastro-intestinal cancers. In Vivo 2017, 31, 1003–1009. [Google Scholar] [PubMed]
- Castro-Marrero, J.; Zaragoza, M.C.; Domingo, J.C.; Martinez-Martinez, A.; Alegre, J.; von Schacky, C. Low omega-3 index and polyunsaturated fatty acid status in patients with chronic fatigue syndrome/myalgic encephalomyelitis. Prostaglandins Leukot. Essent. Fat. Acids 2018, 139, 20–24. [Google Scholar] [CrossRef]
- Aslan, I.; Ozcan, F.; Karaarslan, T.; Kirac, E.; Aslan, M. Decreased eicosapentaenoic acid levels in acne vulgaris reveals the presence of a proinflammatory state. Prostaglandins Other Lipid Mediat. 2017, 128–129, 1–7. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Caruso, M.G.; Bonfiglio, C.; Franco, I.; Mirizzi, A.; De Leonardis, G.; Cozzolongo, R.; Giannuzzi, V.; Giannelli, G.; et al. Aerobic physical activity and a low glycemic diet reduce the aa/epa ratio in red blood cell membranes of patients with nafld. Nutrients 2018, 10, E1299. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizaki, T.; Katsumata, K.; Tsuchida, A.; Wada, T.; Mori, Y.; Hisada, M.; Kawakita, H.; Aoki, T. Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of mmp-9 activity. Int. J. Mol. Med. 2006, 17, 357–362. [Google Scholar] [CrossRef]
- Zuo, X.; Shureiqi, I. Eicosanoid profiling in colon cancer: Emergence of a pattern. Prostaglandins Other Lipid Mediat. 2013, 104–105, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mathew, G.; Jayne, D.G.; Pelengaris, S.; Khan, M. 15-lipoxygenase-1 in colorectal cancer: A review. Tumour Biol. 2009, 30, 185–199. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, D.J.; Marnett, L.J. Cannabinoids, endocannabinoids, and cancer. Cancer Metastasis Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A. The cannabinoid cb(2) receptor: A good friend in the gut. Neurogastroenterol. Motil. 2007, 19, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Yuri, M.; Sasahira, T.; Nakai, K.; Ishimaru, S.; Ohmori, H.; Kuniyasu, H. Reversal of expression of 15-lipoxygenase-1 to cyclooxygenase-2 is associated with development of colonic cancer. Histopathology 2007, 51, 520–527. [Google Scholar] [CrossRef]
- Dixon, D.A.; Blanco, F.F.; Bruno, A.; Patrignani, P. Mechanistic aspects of cox-2 expression in colorectal neoplasia. Recent Results Cancer Res. 2013, 191, 7–37. [Google Scholar]
- Wang, D.; Wang, H.; Shi, Q.; Katkuri, S.; Walhi, W.; Desvergne, B.; Das, S.K.; Dey, S.K.; DuBois, R.N. Prostaglandin e(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell 2004, 6, 285–295. [Google Scholar] [CrossRef]
- Kawamori, T.; Uchiya, N.; Sugimura, T.; Wakabayashi, K. Enhancement of colon carcinogenesis by prostaglandin e2 administration. Carcinogenesis 2003, 24, 985–990. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Smalley, W.E.; DuBois, R.N. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv. Pharmacol. 1997, 39, 1–20. [Google Scholar]
- Nixon, J.B.; Kim, K.S.; Lamb, P.W.; Bottone, F.G.; Eling, T.E. 15-lipoxygenase-1 has anti-tumorigenic effects in colorectal cancer. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 7–15. [Google Scholar] [CrossRef]
- Il Lee, S.; Zuo, X.; Shureiqi, I. 15-lipoxygenase-1 as a tumor suppressor gene in colon cancer: Is the verdict in? Cancer Metastasis Rev. 2011, 30, 481–491. [Google Scholar] [CrossRef]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Kendall, D.A. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010, 215, 611–616. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on ppar activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Sailler, S.; Schmitz, K.; Jager, E.; Ferreiros, N.; Wicker, S.; Zschiebsch, K.; Pickert, G.; Geisslinger, G.; Walter, C.; Tegeder, I.; et al. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience 2014, 1, 272–282. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Valenti, M.; Ligresti, A.; Portella, G.; Di Marzo, V. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004, 18, 1606–1608. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Vandoros, G.P.; Sotiropoulou-Bonikou, G.; Kominea, A.; Papavassiliou, A.G. Nf-kappab/ppar gamma and/or ap-1/ppar gamma ‘on/off’ switches and induction of cbp in colon adenocarcinomas: Correlation with cox-2 expression. Int. J. Colorectal Dis. 2007, 22, 57–68. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Fisk, H.L.; West, A.L.; Childs, C.E.; Burdge, G.C.; Calder, P.C. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; De Leonardis, G.; De Nunzio, V.; Notarnicola, M. Tissue fatty acid profile is differently modulated from olive oil and omega-3 polyunsaturated fatty acids in apcmin/+ mice. Endocr. Metab. Immune Disord. Drug Targets 2017, 17, 303–308. [Google Scholar] [CrossRef]





| CRC Patients (n.68) | ||
|---|---|---|
| No metastases (n = 35) | Metastases (n = 33) | |
| Age | 69.9 ± 13.8 | 68.7 ± 9.3 |
| Sex | ||
| Male | 19 | 22 |
| Female | 16 | 11 |
| Tumor Side | ||
| Right (hepatic flexure, cecum and ascending colon) | 17 | 12 |
| Left (descending colon, sigmoid and rectum) | 18 | 21 |
| Tumor Stage (Clinical staging performed using UICC System) | ||
| Stage I | 5 | 2 |
| Stage II | 24 | 2 |
| Stage III | 5 | 19 |
| Stage IV | 1 | 10 |
| Histological Grading | ||
| Well-differentiated (G1) | 4 | 2 |
| Moderately differentiated (G2) | 20 | 16 |
| Poorly differentiated (G3) | 11 | 15 |
| Metastases Site | ||
| Liver | 0 | 13 |
| Visceral lymph nodes | 0 | 18 |
| Bone | 0 | 1 |
| Lung metastases | 0 | 1 |
| Gene | Primer |
|---|---|
| COX-2 | |
| Forward | 5′-TCTGGTCAATGGAAGCCTGT-3′ |
| Reverse | 5′-CAGCACTTCACGCATCAGTT-3′ |
| 15-LOX-1 | |
| Forward | 5′-CAGACGTGGCTGTGAAAGAC-3′ |
| Reverse | 5′-CAGGAAACCCTCGGTCCTG-3′ |
| CB2-R | |
| Forward | 5′-CCACAACACAACCCAAAGCCT-3′ |
| Reverse | 5′-ATCTCTGTCACCCAGCATTCC-3′ |
| PPAR-γ | |
| Forward | 5′-GGAAGACCACTCGCATTCCTT-3′ |
| Reverse | 5′-GTAATCAGCAACCATTGGGTCA-3′ |
| β-actin | |
| Forward | 5′-AAAGACCTGTACGCCAACACAGTGCTGTCTGG-3′ |
| Reverse | 5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutino, V.; De Nunzio, V.; Caruso, M.G.; Veronese, N.; Lorusso, D.; Di Masi, M.; Benedetto, M.L.; Notarnicola, M. Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. Int. J. Mol. Sci. 2019, 20, 2050. https://doi.org/10.3390/ijms20082050
Tutino V, De Nunzio V, Caruso MG, Veronese N, Lorusso D, Di Masi M, Benedetto ML, Notarnicola M. Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. International Journal of Molecular Sciences. 2019; 20(8):2050. https://doi.org/10.3390/ijms20082050
Chicago/Turabian StyleTutino, Valeria, Valentina De Nunzio, Maria Gabriella Caruso, Nicola Veronese, Dionigi Lorusso, Marta Di Masi, Maria Lucrezia Benedetto, and Maria Notarnicola. 2019. "Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients" International Journal of Molecular Sciences 20, no. 8: 2050. https://doi.org/10.3390/ijms20082050
APA StyleTutino, V., De Nunzio, V., Caruso, M. G., Veronese, N., Lorusso, D., Di Masi, M., Benedetto, M. L., & Notarnicola, M. (2019). Elevated AA/EPA Ratio Represents an Inflammatory Biomarker in Tumor Tissue of Metastatic Colorectal Cancer Patients. International Journal of Molecular Sciences, 20(8), 2050. https://doi.org/10.3390/ijms20082050