Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeño VS-535 Maize Cultivar
Abstract
:1. Introduction
2. Results
2.1. Morphology of Dedifferentiated Tissues
2.2. Plant Regeneration from Tuxpeño VS-535 EC
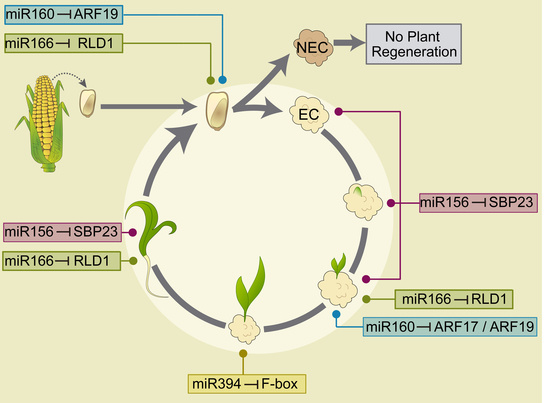
2.3. miRNA and Target Regulation in Tissues with Different Embryogenic Potential
2.4. Embryogenesis-Related miRNA and Target Regulation During Plant Regeneration
3. Discussion
4. Material and Methods
4.1. Callus Induction and Subculture
4.2. Plant Regeneration
4.3. Morphological Characterization
4.4. Scanning Electron Microscopy
4.5. Histological Analysis
4.6. RNA Isolation
4.7. qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugimoto, K.; Gordon, S.P.; Meyerowitz, E.M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Verdeil, J.L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Duclercq, J.; Assoumou Ndong, Y.P.; Guerineau, F.; Sangwan, R.S.; Catterou, M. Arabidopsis shoot organogenesis is enhanced by an amino acid change in the ATHB15 transcription factor. Plant Biol. 2011, 13, 317–324. [Google Scholar] [CrossRef]
- Green, C.E.; Phillips, R.L. Plant Regeneration from Tissue Cultures of Maize. Crop Sci. 1975, 15, 417–421. [Google Scholar] [CrossRef]
- Lu, C.; Vasil, I.K.; Ozias-Akins, P. Somatic embryogenesis in Zea mays L. Theor. Appl. Genet. 1982, 62, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Welter, M.E.; Clayton, D.S.; Miller, M.A.; E Petolino, J. Morphotypes of friable embryogenic maize callus. Plant Cell Rep. 1995, 14, 725–729. [Google Scholar] [PubMed]
- Marín-Méndez, W.; Sanchéz-Chacón, E.; Gatica-Arias, A.M.; Ramírez-Fonseca, P.; Freer-Bustamante, E.; Valdez-Melara, M. Ultrastructure and histology of organogenesis induced from shoot tips of maize (Zea mays, Poaceae). Rev. Biol. Trop. 2009, 57, 129–139. [Google Scholar]
- Rakshit, S.; Rashid, Z.; Sekhar, J.C.; Fatma, T.; Dass, S. Callus induction and whole plant regeneration in elite Indian maize (Zea mays L.) inbreds. Plant Cell. Tissue Organ Cult. 2009, 100, 31–37. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, Z.; Yao, X.; Zhang, Z.; Lin, H.; Zhao, M.; Liu, H.; Peng, H.; Li, S.; Pan, G. Genome expression profile analysis of the immature maize embryo during dedifferentiation. PLoS ONE 2012, 7, e32237. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Sánchez, F. De las variedades criollas de maíz (Zea mays L.) a los híbridos transgénicos. I: Recolección de germoplasma y variedades mejoradas. Agric. Soc. y Desarro. 2008, 5, 151–166. [Google Scholar]
- Garrocho-Villegas, V.; de Jesús-Olivera, M.T.; Quintanar, E.S. Maize somatic embryogenesis: Recent features to improve plant regeneration. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 173–182. ISBN 9781617798. [Google Scholar]
- González, G.A.; Pacheco, M.G.; Oneto, C.D.; Etchart, V.J.; Kandus, M.V.; Salerno, J.C.; Eyherabide, G.; Presello, D.; Lewi, D.M. Somatic embryogenesis and plant regeneration capacity in Argentinean maize (Zea mays L.) inbred lines. Electron. J. Biotechnol. 2012, 15, 9. [Google Scholar]
- Meng, L.; Zhang, S.; Lemaux, P.G. Toward molecular understanding of in vitro and in planta shoot organogenesis. CRC. Crit. Rev. Plant Sci. 2010, 29, 108–122. [Google Scholar] [CrossRef]
- Vashisht, D.; Nodine, M.D. MicroRNA functions in plant embryos: Figure 1. Biochem. Soc. Trans. 2014, 42, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gautam, V.; Singh, S.; Sarkar Das, S.; Verma, S.; Mishra, V.; Mukherjee, S.; Sarkar, A.K. Plant small RNAs: Advancement in the understanding of biogenesis and role in plant development. Planta 2018, 248, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Nodine, M.D.; Bartel, D.P. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev. 2010, 24, 2678–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seefried, W.F.; Willmann, M.R.; Clausen, R.L.; Jenik, P.D. Global Regulation of Embryonic Patterning in Arabidopsis by MicroRNAs. Plant Physiol. 2014, 165, 670–687. [Google Scholar] [CrossRef]
- Zhang, T.-Q.; Lian, H.; Tang, H.; Dolezal, K.; Zhou, C.-M.; Yu, S.; Chen, J.-H.; Chen, Q.; Liu, H.; Ljung, K.; et al. An Intrinsic MicroRNA Timer Regulates Progressive Decline in Shoot Regenerative Capacity in Plants. Plant Cell Online 2015, 27, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Szyrajew, K.; Bielewicz, D.; Dolata, J.; Wójcik, A.M.; Nowak, K.; Szczygieł-Sommer, A.; Szweykowska-Kulinska, Z.; Jarmolowski, A.; Gaj, M.D. MicroRNAs are intensively regulated during induction of somatic embryogenesis in arabidopsis. Front. Plant Sci. 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Liu, Q.; Zhang, Y.C.; Qu, L.H.; Chen, Y.Q.; Gautheret, D. Genome-wide discovery and analysis of microRNAs and other small RNAs from rice embryogenic callus. RNA Biol. 2011, 8, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Chen, J.; Xu, H.; Dong, Z.; Chen, F.; Cui, D. Identification and comparative analysis of microRNA in wheat (triticum aestivum L.) callus derived from mature and immature embryos during In vitro culture. Front. Plant Sci. 2016, 7, 1302. [Google Scholar] [CrossRef] [PubMed]
- Alejandri-Ramírez, N.D.; Chávez-Hernández, E.C.; Contreras-Guerra, J.L.; Reyes, J.L.; Dinkova, T.D. Small RNA differential expression and regulation in Tuxpeño maize embryogenic callus induction and establishment. Plant Physiol. Biochem. 2018, 122, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Hernández, E.C.; Alejandri-Ramírez, N.D.; Juárez-González, V.T.; Dinkova, T.D. Maize miRNA and target regulation in response to hormone depletion and light exposure during somatic embryogenesis. Front. Plant Sci. 2015, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.K.; Kubo, M.; Zhong, R.; Demura, T.; Ye, Z.H. Overexpression of miR165 affects apical meristem formation, organ polarity establishment and vascular development in Arabidopsis. Plant Cell Physiol. 2007, 48, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, C.; Xu, J. Control of Cell Fate Reprogramming Towards de Novo Shoot Organogenesis. Plant Cell Physiol. 2018, 59, 713–719. [Google Scholar] [CrossRef]
- Qiao, M.; Zhao, Z.; Song, Y.; Liu, Z.; Cao, L.; Yu, Y.; Li, S.; Xiang, F. Proper regeneration from in vitro cultured Arabidopsis thaliana requires the microRNA-directed action of an auxin response factor. Plant J. 2012, 71, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, C.L.; Green, C.E. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 1985, 164, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Su, S.; Wu, Y.; Li, Y.; Shan, X.; Li, S.; Liu, H.; Dong, H.; Ding, M.; Han, J.; et al. Histological and transcript analyses of intact somatic embryos in an elite maize (Zea mays L.) inbred line Y423. Plant Physiol. Biochem. 2015, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jiang, Z.; Lu, S.; Lin, H.; Gao, S.; Peng, H.; Yuan, G.; Liu, L.; Zhang, Z.; Zhao, M.; et al. Combined small RNA and degradome sequencing reveals microRNA regulation during immature maize embryo dedifferentiation. Biochem. Biophys. Res. Commun. 2013, 44, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qin, C.; Chen, Z.; Zuo, T.; Yang, X.; Zhou, H.; Xu, M.; Cao, S.; Shen, Y.; Lin, H.; et al. Identification of miRNAs and their target genes in developing maize ears by combined small RNA and degradome sequencing. BMC Genomics 2014, 15, 25. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, J.; Yuan, H.; McGinnis, C. Distinct tissue-specific transcriptional regulation revealed by gene regulatory networks in maize. BMC Plant Biology 2018, 18, 111. [Google Scholar] [CrossRef]
- Conger, B.V.; Novak, F.J.; Afza, R.; Erdelsky, K. Somatic embryogenesis from cultured leaf segments of Zea mays. Plant Cell Rep. 1987, 6, 345–347. [Google Scholar] [CrossRef]
- Emons, A.M.C.; Kieft, H. Somatic Embryogenesis in Maize (Zea mays L.). In Somatic Embryogenesis and Synthetic Seed II; Springer: Berlin/Heidelberg, Germany, 1995; p. 444. ISBN 978-3-642-78643-3. [Google Scholar]
- Anami, S.E.; Mgutu, A.J.; Taracha, C.; Coussens, G.; Karimi, M.; Hilson, P.; Van Lijsebettens, M.; Machuka, J. Somatic embryogenesis and plant regeneration of tropical maize genotypes. Plant Cell. Tissue Organ Cult. 2010, 102, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Garrocho-Villegas, V.; Aguilar, R.; de Jiménez, E.S. Contribution of the Zea mays insulin-like growth factor (ZmIGF) to the embryogenic competence of maize tissue cultures. Vitr. Cell. Dev. Biol. Plant 2017, 53, 122–132. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Y.; Su, S.; Liu, H.; Yang, G.; Li, S.; Shan, X.; Yuan, Y. Differential gene expression during somatic embryogenesis in the maize (Zea mays L.) inbred line H99. Plant Cell. Tissue Organ Cult. 2012, 109, 271–286. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Y.; Zou, H.; Su, S.; Li, S.; Shan, X.; Xi, J.; Yuan, Y. Comparative proteomic analysis of the H99 inbred maize (Zea mays L.) line in embryogenic and non-embryogenic callus during somatic embryogenesis. Plant Cell. Tissue Organ Cult. 2013, 113, 103–119. [Google Scholar] [CrossRef]
- Varhaníková, M.; Uvackova, L.; Skultety, L.; Pretova, A.; Obert, B.; Hajduch, M. Comparative quantitative proteomic analysis of embryogenic and non-embryogenic calli in maize suggests the role of oxylipins in plant totipotency. J. Proteomics 2014, 104, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; La Rota, M.; Hoerster, G.; Hastings, C.; Wang, N.; Chamberlin, M.; Wu, E.; Jones, T.; Gordon-Kamm, W. Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. Vitr. Cell. Dev. Biol. Plant 2018, 28, 1998–2015. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhang, X.S. Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis. Plant Signal. Behav. 2009, 4, 574–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Chen, Y.Q.; Liu, Q. Sculpting the meristem: The roles of miRNAs in plant stem cells. Biochem. Biophys. Res. Commun. 2011, 17, 2204–2216. [Google Scholar] [CrossRef]
- Knauer, S.; Holt, A.L.; Rubio-Somoza, I.; Tucker, E.J.; Hinze, A.; Pisch, M.; Javelle, M.; Timmermans, M.C.; Tucker, M.R.; Laux, T. A Protodermal miR394 Signal Defines a Region of Stem Cell Competence in the Arabidopsis Shoot Meristem. Dev. Cell 2013, 24, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Q.; Lian, H.; Zhou, C.-M.; Xu, L.; Jiao, Y.; Wang, J.-W. A Two-Step Model for de Novo Activation of WUSCHEL during Plant Shoot Regeneration. Plant Cell 2017, 29, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.; Whipple, C.; Jackson, D.; Hake, S. The maize SBP-box transcription factor encoded by tasselsheath4 regulates bract development and the establishment of meristem boundaries. Development 2010, 137, 1243–1250. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.D.; Yu, L.J.; Li, Z.J.; Yan, Y.; Han, R.; Liu, H.; Ma, M. Genome-wide analysis of the SPL family transcription factors and their responses to abiotic stresses in maize. Plant Gene 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Long, J.M.; Liu, C.Y.; Feng, M.Q.; Liu, Y.; Wu, X.M.; Guo, W.W. MiR156-SPL modules regulate induction of somatic embryogenesis in citrus callus. J. Exp. Bot. 2018, 69, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Laufs, P. MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 2004, 131, 4311–4322. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.; Ohme-Takagi, M. TCPs, WUSs, and WINDs: Families of transcription factors that regulate shoot meristem formation, stem cell maintenance, and somatic cell differentiation. Front. Plant Sci. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Cheng, Z.J.; Sang, Y.L.; Zhang, M.M.; Rong, X.F.; Wang, Z.W.; Tang, Y.Y.; Zhang, X.S. Type-B ARABIDOPSIS RESPONSE REGULATORs Is Critical to the Specification of Shoot Stem Cell Niche by Dual Regulation of WUSCHEL. Plant Cell 2017, 29, 1357–1372. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.-J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef] [PubMed]
- Sultana, M.; Gangopadhyay, G. Early expression of WUSCHEL is a marker for in vitro shoot morphogenesis in tobacco and Beta palonga. Plant Cell. Tissue Organ Cult. 2018, 134, 277–288. [Google Scholar] [CrossRef]
- Nogueira, F.T.S.; Chitwood, D.H.; Madi, S.; Ohtsu, K.; Schnable, P.S.; Scanlon, M.J.; Timmermans, M.C.P. Regulation of small RNA accumulation in the maize shoot apex. PLoS Genet. 2009, 5, e1000320. [Google Scholar] [CrossRef] [PubMed]
- Carlsbecker, A.; Lee, J.Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vatén, A.; Thitamadee, S.; et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-W. Control of Root Cap Formation by MicroRNA-Targeted Auxin Response Factors in Arabidopsis. Plant Cell Online 2005, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Jones-Rhoades, M.W.; Bartel, D.P. Computational identification of plant MicroRNAs and their targets, including a stress-induced miRNA. Mol. Cell. 2004, 14, 787–799. [Google Scholar] [CrossRef]
- Brodersen, P.; Sakvarelidze-Achard, L.; Bruun-Rasmussen, M.; Dunoyer, P.; Yamamoto, Y.Y.; Sieburth, L.; Voinnet, O. Widespread translational inhibition by plant miRNAs and siRNAs. Science 2008, 320, 1185–1190. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic Plasticity of Adventitious Rooting in Arabidopsis Is Controlled by Complex Regulation of AUXIN RESPONSE FACTOR Transcripts and MicroRNA Abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Gaj, M.D. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017, 36, 843–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, R.S.; Lin, H.; Childs, K.L.; Hansey, C.N.; Robin Buell, C.; De Leon, N.; Kaeppler, S.M. Genome-wide atlas of transcription during maize development. Plant J. 2011, 66, 553–563. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Chávez-Hernández, E.C.; Dinkova, T.D. MicroRNA Expression and Regulation During Maize Somatic Embryogenesis. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; pp. 397–410. ISBN 9781493985944. [Google Scholar]
- Sandoval, E. Técnicas aplicadas al estudio de la anatomía vegetal; UNAM Instituto de Biología: D.F., México, 2005; p. 278. ISBN 9789703231317. [Google Scholar]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 8, 538–547. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ruiz, B.A.; Juárez-González, V.T.; Sandoval-Zapotitla, E.; Dinkova, T.D. Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeño VS-535 Maize Cultivar. Int. J. Mol. Sci. 2019, 20, 2079. https://doi.org/10.3390/ijms20092079
López-Ruiz BA, Juárez-González VT, Sandoval-Zapotitla E, Dinkova TD. Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeño VS-535 Maize Cultivar. International Journal of Molecular Sciences. 2019; 20(9):2079. https://doi.org/10.3390/ijms20092079
Chicago/Turabian StyleLópez-Ruiz, Brenda A., Vasti T. Juárez-González, Estela Sandoval-Zapotitla, and Tzvetanka D. Dinkova. 2019. "Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeño VS-535 Maize Cultivar" International Journal of Molecular Sciences 20, no. 9: 2079. https://doi.org/10.3390/ijms20092079
APA StyleLópez-Ruiz, B. A., Juárez-González, V. T., Sandoval-Zapotitla, E., & Dinkova, T. D. (2019). Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeño VS-535 Maize Cultivar. International Journal of Molecular Sciences, 20(9), 2079. https://doi.org/10.3390/ijms20092079