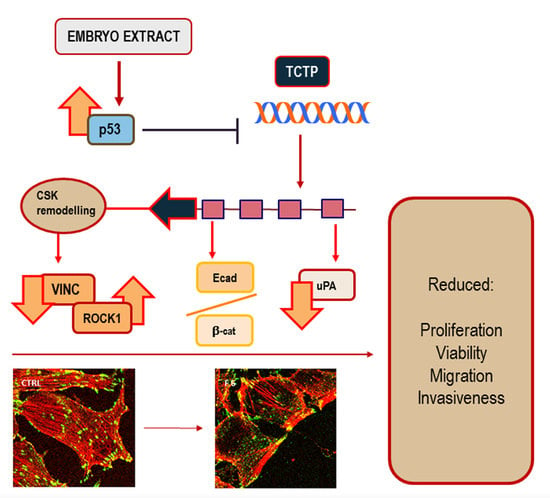
Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-Regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway
Abstract
:1. Introduction
2. Results
2.1. Recognizing the Most Effective Embryo Fish Extract Fraction
2.2. Embryo Extract Stimulates Apoptosis
2.3. Embryo Extract Reduces Cancer Cell Proliferation
2.4. Embryo Extract Antagonizes Cancer Cell Invasiveness and Migrating Capability
2.5. Cytoskeleton Remodeling
2.6. Embryo Extracts Promotes E-cadherin/β-catenin Redistribution behind Cell Membrane
2.7. Embryo Extracts Downregulate TCTP Expression in MDA-MB-231 Cells
3. Discussion
4. Materials and Methods
4.1. Experimental Cell Model
4.2. Zebrafish Embryo Extracts
4.3. In Vitro Toxicology Assay Kit Sulforhodamine B Based
4.4. Cell Migration Assay
4.5. Cell Invasion Assay
4.6. Cell Proliferation
4.7. Muse™ Annexin V & Dead Cell Kit
4.8. Western Blots
4.9. Densitometry
4.10. Confocal Microscopy
4.11. Urokinase-PA Zimography
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Joel, M.; Sandberg, C.J.; Boulland, J.L.; Vik-Mo, E.O.; Langmoen, I.A.; Glover, J.C. Inhibition of tumor formation and redirected differentiation of glioblastoma cells in a xenotypic embryonic environment. Dev. Dyn. 2013, 242, 1078–1093. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Kasemeier-Kulesa, J.; Kulesa, P.M.; Postovit, L.M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat. Rev. Cancer 2007, 7, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Mintz, B.; Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. USA 1975, 72, 3585–3589. [Google Scholar] [CrossRef]
- Bizzarri, M.; Cucina, A.; Biava, P.M.; Proietti, S.; D’Anselmi, F.; Dinicola, S.; Pasqualato, A.; Lisi, E. Embryonic morphogenetic field induces phenotypic reversion in cancer cells. Curr. Pharm. Biotechnol. 2011, 12, 243–253. [Google Scholar] [CrossRef]
- Biava, P.M.; Canaider, S.; Facchin, F.; Bianconi, E.; Ljungberg, L.; Rotilio, D.; Burigana, F.; Ventura, C. Stem Cell Differentiation Stage Factors from Zebrafish Embryo: A Novel Strategy to Modulate the Fate of Normal and Pathological Human (Stem) Cells. Curr. Pharm. Biotechnol. 2015, 16, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, D.; Rogers, I.M.; Nagy, A.; Calogero, A.E.; Brown, T.J.; Casper, R.F. Human embryonic stem cells secrete soluble factors that inhibit cancer cell growth. Cell Prolif. 2009, 42, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Hansis, C.; Barreto, G.; Maltry, N.; Niehrs, C. Nuclear reprogramming of human somatic cells by xenopus egg extract requires BRG1. Curr. Biol. 2004, 14, 1475–1480. [Google Scholar] [CrossRef]
- Ferranti, F.; D’Anselmi, F.; Caruso, M.; Lei, V.; Dinicola, S.; Pasqualato, A.; Cucina, A.; Palombo, A.; Ricci, G.; Catizone, A.; et al. TCam-2 seminoma cells exposed to egg-derived microenvironment modify their shape, adhesive pattern and migratory behaviour: A molecular and morphometric analysis. PLoS ONE 2013, 8, e76192. [Google Scholar] [CrossRef]
- D’Anselmi, F.; Masiello, M.G.; Cucina, A.; Proietti, S.; Dinicola, S.; Pasqualato, A.; Ricci, G.; Dobrowolny, G.; Catizone, A.; Palombo, A.; et al. Microenvironment promotes tumor cell reprogramming in human breast cancer cell lines. PLoS ONE 2013, 8, e83770. [Google Scholar] [CrossRef] [PubMed]
- Allegrucci, C.; Rushton, M.D.; Dixon, J.E.; Sottile, V.; Shah, M.; Kumari, R.; Watson, S.; Alberio, R.; Johnson, A.D. Epigenetic reprogramming of breast cancer cells with oocyte extracts. Mol. Cancer 2011, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Abollo-Jiménez, F.; Jiménez, R.; Cobaleda, C. Physiological cellular reprogramming and cancer. Semin. Cancer Biol. 2010, 20, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Maffini, M.V.; Sonnenschein, C. Neoplasia as development gone awry: The role of endocrine disruptors. Int. J. Androl. 2008, 31, 288–293. [Google Scholar] [CrossRef]
- Cucina, A.; Biava, P.; D’Anselmi, F.; Coluccia, P.; Conti, F.; di Clemente, R.; Miccheli, A.; Frati, L.; Gulino, A.; Bizzarri, M. Zebrafish embryo proteins induce apoptosis in human colon cancer cells. Apoptosis 2006, 11, 1617–1628. [Google Scholar] [CrossRef]
- Astigiano, S.; Damonte, P.; Fossati, S.; Boni, L.; Barbieri, O. Fate of embryonal carcinoma cells injected into postimplantation mouse embryos. Differentiation 2005, 73, 484–490. [Google Scholar] [CrossRef]
- Tabata, T.; Takei, Y. Morphogens, their identification and regulation. Development 2004, 131, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Maffini, M.V.; Soto, A.M.; Sonnenschein, C. The microenvironment determines the breast cancer cells’ phenotype: Organization of MCF7 cells in 3D cultures. BMC Cancer 2010, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Downing, T.L.; Soto, J.; Morez, C.; Houssin, T.; Fritz, A.; Yuan, F.; Chu, J.; Patel, S.; Schaffer, D.V.; Li, S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013, 12, 1154–1162. [Google Scholar] [CrossRef]
- D’Anselmi, F.; Cucina, A.; Biava, P.M.; Proietti, S.; Coluccia, P.; Frati, L.; Bizzarri, M. Zebrafish stem cell differentiation stage factors suppress Bcl-xL release and enhance 5-Fu-mediated apoptosis in colon cancer cells. Curr. Pharm. Biotechnol. 2011, 12, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Meloni, F.; Frosi, A.; Lazzaroni, S.; Bizzarri, M.; Frati, L.; Biava, P.M. Treatment with stem cell differentiation stage factors of intermediate-advanced hepatocellular carcinoma: An open randomized clinical trial. Oncol. Res. 2005, 15, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Cucina, A.; Giuliani, A.; Verna, R.; Palombi, E.; Biava, P.M.; Pensotti, A. Fish protein extract enhances clinical response to salvage chemotherapy in colon cancer patients. Org. J. Biol. Sci. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Pfeiffer, M.J.; Siatkowski, M.; Paudel, Y.; Balbach, S.T.; Baeumer, N.; Crosetto, N.; Drexler, H.C.; Fuellen, G.; Boiani, M. Proteomic analysis of mouse oocytes reveals 28 candidate factors of the “reprogrammome”. J. Proteome Res. 2011, 10, 2140–2153. [Google Scholar] [CrossRef]
- Bischof, A.G.; Yüksel, D.; Mammoto, T.; Mammoto, A.; Krause, S.; Ingber, D.E. Breast cancer normalization induced by embryonic mesenchyme is mediated by extracellular matrix biglycan. Integr. Biol. 2013, 5, 1045–1056. [Google Scholar] [CrossRef]
- DeCosse, J.J.; Gossens, C.L.; Kuzma, J.F.; Unsworth, B.R. Breast cancer: Induction of differentiation by embryonic tissue. Science 1973, 181, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, S.; Sturtzel, C.; Pascoal, S.; Distel, M. Quo natas, Danio? Recent Progress in Modeling Cancer in Zebrafish. Front. Oncol. 2017, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, R.B.; Pines, D.; Schmalian, J.; Stojković, B.P.; Wolynes, P. The middle way. Proc. Natl. Acad. Sci. USA 2000, 97, 32–37. [Google Scholar] [CrossRef]
- Bertolaso, M.; Bizzarri, M.; Pensotti, A.; Giuliani, A. Co-Emergence and Collapse: The Mesoscopic Approach for Conceptualizing and Investigating the Functional Integration of Organisms. Front. Phisiol. 2019, in press. [Google Scholar]
- Moirangthem, A.; Bondhopadhyay, B.; Mukherjee, M.; Bandyopadhyay, A.; Mukherjee, N.; Konar, K.; Bhattacharya, S.; Basu, A. Simultaneous knockdown of uPA and MMP9 can reduce breast cancer progression by increasing cell-cell adhesion and modulating EMT genes. Sci. Rep. 2016, 24, 21903. [Google Scholar] [CrossRef]
- Simeoni, C.; Dinicola, S.; Cucina, A.; Mascia, C.; Bizzarri, M. Systems Biology Approach and Mathematical Modeling for Analyzing Phase-Space Switch During Epithelial-Mesenchymal Transition. Methods Mol. Biol. 2018, 1702, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Diez, G.; Koch, T.M.; Marg, S.; Ziegler, W.H.; Goldmann, W.H.; Fabry, B. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J. Biol. Chem. 2010, 285, 13121–13130. [Google Scholar] [CrossRef]
- Saunders, R.M.; Holt, M.R.; Jennings, L.; Sutton, D.H.; Barsukov, I.L.; Bobkov, A.; Liddington, R.C.; Adamson, E.A.; Dunn, G.A.; Critchley, D.R. Role of vinculin in regulating focal adhesion turnover. Eur. J. Cell Biol. 2006, 85, 487–500. [Google Scholar] [CrossRef]
- Breyer, J.; Samarin, J.; Rehm, M.; Lautscham, L.; Fabry, B.; Goppelt-Struebe, M. Inhibition of Rho kinases increases directional motility of microvascular endothelial cells. Biochem. Pharmacol. 2012, 83, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Mavria, G.; Vercoulen, Y.; Yeo, M.; Paterson, H.; Karasarides, M.; Marais, R.; Bird, D.; Marshall, C.J. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 2006, 9, 33–44. [Google Scholar] [CrossRef]
- Sivasubramaniyan, K.; Pal, R.; Totey, S.; Bhat, V.S.; Totey, S. Rho kinase inhibitor y27632 alters the balance between pluripotency and early differentiation events in human embryonic stem cells. Curr. Stem Cell Res. Ther. 2010, 5, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Laeno, A.M.; Tamashiro, D.A.; Alarcon, V.B. Rho-Associated Kinase Activity Is Required for Proper Morphogenesis of the Inner Cell Mass in the Mouse Blastocyst. Biol. Reprod. 2013, 89, 122. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; LaMonica, K.; Hong, T.; Pagliaruli, T.; Mulrooney, J.; Grabel, L. Roles for Rho/ROCK and vinculin in parietal endoderm migration. Cell Commun. Adhes. 2005, 12, 9–22. [Google Scholar] [CrossRef]
- Grille, S.J.; Bellacosa, A.; Upson, J.; Klein-Szanto, A.J.; van Roy, F.; Lee-Kwon, W.; Donowitz, M.; Tsichlis, P.N.; Larue, L. The protein kinase Akt induces epithelial-mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003, 63, 2172–2178. [Google Scholar]
- Orsulic, S.; Huber, O.; Aberle, H.; Arnold, S.; Kemler, R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell Sci. 1999, 112, 1237–1245. [Google Scholar] [PubMed]
- Tuynder, M.; Susini, L.; Prieur, S.; Besse, S.; Fiucci, G.; Amson, R.; Telerman, A. Biological models and genes of tumor reversion: Cellular reprogramming through tpt1/TCTP and SIAH-1. Proc. Natl. Acad. Sci. USA 2002, 99, 14976–14981. [Google Scholar] [CrossRef]
- Rho, S.B.; Lee, J.H.; Park, M.S.; Byun, H.J.; Kang, S.; Seo, S.S.; Kim, J.Y.; Park, S.Y. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011, 585, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Kawaguchi, Y.; Lai, C.H.; Kovacs, J.J.; Higashimoto, Y.; Appella, E.; Yao, T.P. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002, 21, 6236–6245. [Google Scholar] [CrossRef]
- Amson, R.; Pece, S.; Lespagnol, A.; Vyas, R.; Mazzarol, G.; Tosoni, D.; Colaluca, I.; Viale, G.; Rodrigues-Ferreira, S.; Wynendaele, J.; et al. Reciprocal repression between P53 and TCTP. Nat. Med. 2011, 18, 91–99. [Google Scholar] [CrossRef]
- Skrypek, N.; Goossens, S.; De Smedt, E.; Vandamme, N.; Berx, G. Epithelial-to-Mesenchymal Transition: Epigenetic Reprogramming Driving Cellular Plasticity. Trends Genet. 2017, 33, 943–959. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.G. The paradox of E2F1: Oncogene and tumor suppressor gene. Mol. Carcinog. 2000, 27, 151–157. [Google Scholar] [CrossRef]
- Kawamura, T.; Suzuki, J.; Wang, Y.V.; Menendez, S.; Morera, L.B.; Raya, A.; Wahl, G.M.; Izpisúa Belmonte, J.C. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature 2009, 460, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Lowe, S.W. Stem cells: The promises and perils of p53. Nature 2009, 460, 1085–1086. [Google Scholar] [CrossRef]
- Amson, R.; Karp, J.E.; Telerman, A. Lessons from tumor reversion for cancer treatment. Curr. Opin. Oncol. 2013, 25, 59–65. [Google Scholar] [CrossRef]
- Biava, P.M.; Carluccio, A. Activation of anti-oncogene p53 produced by embryonic extracts in vitro tumor cells. J. Tumor Marker Oncol. 1997, 12, 9–15. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proietti, S.; Cucina, A.; Pensotti, A.; Biava, P.M.; Minini, M.; Monti, N.; Catizone, A.; Ricci, G.; Leonetti, E.; Harrath, A.H.; et al. Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-Regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway. Int. J. Mol. Sci. 2019, 20, 2151. https://doi.org/10.3390/ijms20092151
Proietti S, Cucina A, Pensotti A, Biava PM, Minini M, Monti N, Catizone A, Ricci G, Leonetti E, Harrath AH, et al. Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-Regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway. International Journal of Molecular Sciences. 2019; 20(9):2151. https://doi.org/10.3390/ijms20092151
Chicago/Turabian StyleProietti, Sara, Alessandra Cucina, Andrea Pensotti, Pier Mario Biava, Mirko Minini, Noemi Monti, Angela Catizone, Giulia Ricci, Erica Leonetti, Abdel Halim Harrath, and et al. 2019. "Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-Regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway" International Journal of Molecular Sciences 20, no. 9: 2151. https://doi.org/10.3390/ijms20092151
APA StyleProietti, S., Cucina, A., Pensotti, A., Biava, P. M., Minini, M., Monti, N., Catizone, A., Ricci, G., Leonetti, E., Harrath, A. H., Alwasel, S. H., & Bizzarri, M. (2019). Active Fraction from Embryo Fish Extracts Induces Reversion of the Malignant Invasive Phenotype in Breast Cancer through Down-Regulation of TCTP and Modulation of E-cadherin/β-catenin Pathway. International Journal of Molecular Sciences, 20(9), 2151. https://doi.org/10.3390/ijms20092151