Multikinase Inhibitor Treatment in Thyroid Cancer
Abstract
:1. Introduction
2. Thyroid Cancer
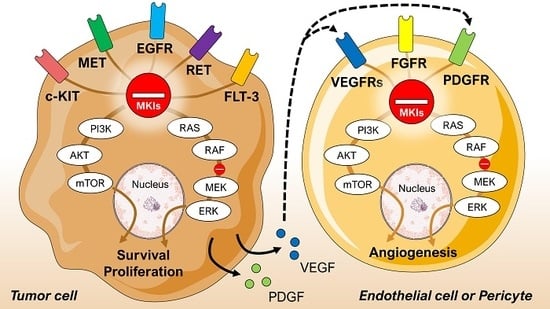
3. Multikinase Inhibitors
4. Methods
4.1. Eligibility Criteria
4.2. Information Sources
4.3. Search
4.4. Study Selection
4.5. Data Collection Process
4.6. Data Items
4.7. Risk of Bias in Individual Studies
5. Results
6. Discussion
7. Conclusions
8. Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AE(s) | Adverse effect(s) |
| AKT | Protein kinase B |
| ATC | Anaplastic thyroid cancer |
| c-KIT | Stem cell factor receptor |
| DTC | Differentiated thyroid cancer |
| EGFR | Epidermal growth factor receptor |
| ERK | Mitogen-activated protein kinase |
| FGFR | Fibroblast growth factor receptor |
| FLT-3 | FMS-like tyrosine kinase 3 |
| FTC | Follicular thyroid cancer |
| HCC | Hürthle cell carcinoma |
| HIF | Hypoxia-inducible factor |
| MEK | Mitogen-activated protein kinase kinase |
| MET | Hepatocyte growth factor receptor |
| MKI(s) | Multikinase inhibitor(s) |
| MTC | Medullary thyroid cancer |
| mTOR | Mammalian target of rapamycin |
| N/A | Not available |
| N/R | Not reached |
| OS | Overall survival |
| PDGFR | Platelet-derived growth factor receptor |
| PDTC(s) | Poorly differentiated thyroid cancer(s) |
| PFS | Progression-free survival |
| PGF | Placental growth factor |
| PI3K | phosphoinositide 3-kinase |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PTC | Papillary thyroid cancer |
| RAF | Rapidly accelerated fibrosarcoma kinase |
| RAI | Radioactive iodine |
| RAS | Rat sarcoma protein |
| RET | Rearranged during transfection |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RR | Response rate |
| TSH | Thyroid-stimulating hormone |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| VHL | Von Hippel–Lindau tumor suppressor |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirian, C.; Gronhoj, C.; Jensen, D.H.; Jakobsen, K.K.; Karnov, K.; Jensen, J.S.; Hahn, C.H.; Klitmoller, T.A.; Bentzen, J.; von Buchwald, C. Trends in thyroid cancer: Retrospective analysis of incidence and survival in denmark 1980–2014. Cancer Epidemiol. 2018, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.J.; Oucharek, J.; Learoyd, D.; Sidhu, S.B. Standard and emerging therapies for metastatic differentiated thyroid cancer. Oncologis 2010, 15, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceolin, L.; Duval, M.; Benini, A.F.; Ferreira, C.V.; Maia, A.L. Medullary thyroid carcinoma beyond surgery: Advances, challenges, and perspectives. Endocr. Relat. Cancer 2019, 26, R499–R518. [Google Scholar] [CrossRef] [Green Version]
- Kapiteijn, E.; Schneider, T.C.; Morreau, H.; Gelderblom, H.; Nortier, J.W.; Smit, J.W. New treatment modalities in advanced thyroid cancer. Ann. Oncol. 2012, 23, 10–18. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Volante, M.; Collini, P.; Nikiforov, Y.E.; Sakamoto, A.; Kakudo, K.; Katoh, R.; Lloyd, R.V.; LiVolsi, V.A.; Papotti, M.; Sobrinho-Simoes, M.; et al. Poorly differentiated thyroid carcinoma: The turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am. J. Surg. Pathol. 2007, 31, 1256–1264. [Google Scholar] [CrossRef]
- Tiedje, V.; Schmid, K.W.; Weber, F.; Bockisch, A.; Fuhrer, D. Differentiated thyroid cancer. Internist 2015, 56, 153–166. [Google Scholar] [CrossRef]
- Stjepanovic, N.; Capdevila, J. Multikinase inhibitors in the treatment of thyroid cancer: Specific role of lenvatinib. Biol. Targets Ther. 2014, 8, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.Y.; Busaidy, N.L. Treatment and surveillance of advanced, metastatic iodine-resistant differentiated thyroid cancer. Curr. Opin. Oncol. 2017, 29, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar] [PubMed]
- Cabanillas, M.E.; Takahashi, S. Managing the adverse events associated with lenvatinib therapy in radioiodine-refractory differentiated thyroid cancer. In Seminars in Oncology; W.B. Saunders Ltd.: Philadelphia, PA, USA, 2019; Volume 46, pp. 57–64. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivee, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by vegf receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef]
- Kowanetz, M.; Ferrara, N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006, 12, 5018–5022. [Google Scholar] [CrossRef] [Green Version]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase ii study of daily sunitinib in fdg-pet-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.M.; Centanni, M.; Virili, C.; Miccoli, M.; Ferrari, P.; Ruffilli, I.; Ragusa, F.; Antonelli, A.; Fallahi, P. Sunitinib in the treatment of thyroid cancer. Curr. Med. Chem. 2019, 26, 963–972. [Google Scholar] [CrossRef]
- Budolfsen, C.; Faber, J.; Grimm, D.; Krüger, M.; Bauer, J.; Wehland, M.; Infanger, M.; Magnusson, N.E. Tyrosine kinase inhibitor-induced hypertension: Role of hypertension as a biomarker in cancer treatment. Curr. Vasc. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Santini, F.; Corrado, A.; Materazzi, G.; Ulisse, S.; Miccoli, P.; Antonelli, A. Sorafenib and thyroid cancer. BioDrugs 2013, 27, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Thadi, A.; Shailubhai, K. Hepatocellular carcinoma: Etiology and current and future drugs. J. Clin. Exp. Hepatol. 2019, 9, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Liu, P.; Yang, J.; Wu, P.; Chen, B.; Liu, Z.; Li, Z. Evaluation of targeted agents for advanced and unresectable hepatocellular carcinoma: A network meta-analysis. J. Cancer 2019, 10, 4671–4678. [Google Scholar] [CrossRef] [Green Version]
- Milling, R.V.; Grimm, D.; Krüger, M.; Grosse, J.; Kopp, S.; Bauer, J.; Infanger, M.; Wehland, M. Pazopanib, cabozantinib, and vandetanib in the treatment of progressive medullary thyroid cancer with a special focus on the adverse effects on hypertension. Int. J. Mol. Sci. 2018, 19, 3258. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, R.B.; Beijnen, J.H.; Schellens, J.H.M.; Huitema, A.D.R.; Steeghs, N. Clinical pharmacokinetics and pharmacodynamics of pazopanib: Towards optimized dosing. Clin. Pharmacokinet. 2017, 56, 987–997. [Google Scholar] [CrossRef] [Green Version]
- Martinez Chanza, N.; Xie, W.; Asim Bilen, M.; Dzimitrowicz, H.; Burkart, J.; Geynisman, D.M.; Balakrishnan, A.; Bowman, I.A.; Jain, R.; Stadler, W.; et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: A multicentre, retrospective, cohort study. Lancet Oncol. 2019, 20, 581–590. [Google Scholar] [CrossRef]
- Baek Möller, N.; Budolfsen, C.; Grimm, D.; Krüger, M.; Infanger, M.; Wehland, M.; Nils, E.M. Drug-induced hypertension caused by multikinase inhibitors (sorafenib, sunitinib, lenvatinib and axitinib) in renal cell carcinoma treatment. Int. J. Mol. Sci. 2019, 20, 4712. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Litiere, S.; Collette, S.; de Vries, E.G.; Seymour, L.; Bogaerts, J. Recist—Learning from the past to build the future. Nat. Rev. Clin. Oncol. 2017, 14, 187–192. [Google Scholar] [CrossRef]
- Locati, L.D.; Piovesan, A.; Durante, C.; Bregni, M.; Castagna, M.G.; Zovato, S.; Giusti, M.; Ibrahim, T.; Puxeddu, E.; Fedele, G.; et al. Real-world efficacy and safety of lenvatinib: Data from a compassionate use in the treatment of radioactive iodine-refractory differentiated thyroid cancer patients in italy. Eur. J. Cancer 2019, 118, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Yamazaki, H.; Takasaki, H.; Suganuma, N.; Nakayama, H.; Toda, S.; Masudo, K. Lenvatinib as a novel treatment for anaplastic thyroid cancer: A retrospective study. Oncol. Lett. 2018, 16, 7271–7277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Vega, M.; Garcia-Aleman, J.; Sebastian-Ochoa, A.; Mancha-Doblas, I.; Trigo-Perez, J.M.; Tinahones-Madueno, F. Tyrosine kinase inhibitors in iodine-refractory differentiated thyroid cancer: Experience in clinical practice. Endocrine 2018, 59, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Balmelli, C.; Railic, N.; Siano, M.; Feuerlein, K.; Cathomas, R.; Cristina, V.; Guthner, C.; Zimmermann, S.; Weidner, S.; Pless, M.; et al. Lenvatinib in advanced radioiodine-refractory thyroid cancer—A retrospective analysis of the swiss lenvatinib named patient program. J. Cancer 2018, 9, 250–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianoukakis, A.G.; Dutcus, C.E.; Batty, N.; Guo, M.; Baig, M. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr. Relat. Cancer 2018, 25, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.I.; Elisei, R.; Dedecjus, M.; Popovtzer, A.; Druce, M.; Kapiteijn, E.; Pacini, F.; Locati, L.; Krajewska, J.; Weiss, R.; et al. Safety and efficacy of two starting doses of vandetanib in advanced medullary thyroid cancer. Endocr. Relat. Cancer 2019, 26, 241–250. [Google Scholar] [CrossRef]
- Iwasaki, H.; Yamazaki, H.; Takasaki, H.; Suganuma, N.; Sakai, R.; Nakayama, H.; Hatori, S.; Toda, S.; Masudo, K. Treatment outcomes of differentiated thyroid cancer with distant metastasis improve by tyrosine kinase inhibitors. Oncol. Lett. 2019, 17, 5292–5300. [Google Scholar] [CrossRef] [Green Version]
- Jerkovich, F.; Garcia Falcone, M.G.; Pitoia, F. The experience of an endocrinology division on the use of tyrosine multikinase inhibitor therapy in patients with radioiodine-resistant differentiated thyroid cancer. Endocrine 2019, 64, 632–638. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.H.; Shin, D.Y.; Lim, D.J.; Kim, E.Y.; Kim, W.B.; Chung, J.H.; Shong, Y.K.; Kim, B.H.; Kim, W.G. Tertiary care experience of sorafenib in the treatment of progressive radioiodine-refractory differentiated thyroid carcinoma: A korean multicenter study. Thyroid 2018, 28, 340–348. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.M.; Lee, E.K.; Hwangbo, Y.; Lee, Y.J.; Cho, S.W.; Park, D.J.; Lee, Y.; Park, Y.J. Tumor doubling time predicts response to sorafenib in radioactive iodine-refractory differentiated thyroid cancer. Endocr. J. 2019, 66, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, S.M.; Chang, H.; Kim, B.W.; Lee, Y.S.; Chang, H.S.; Park, C.S. Safety of tyrosine kinase inhibitors in patients with differentiated thyroid cancer: Real-world use of lenvatinib and sorafenib in korea. Front. Endocrinol. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, J.; Szekanecz, E.; Bassam, A.; Uhlyarik, A.; Papai, Z.; Rubovszky, G.; Mezosi, E.; Rucz, K.; Garai, I.; Nagy, E.; et al. First line sorafenib treatment for metastatic medullary thyroid cancer: Efficacy and safety analysis. Exp. Clin. Endocrinol. Diabetes 2019, 127, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Miyake, N.; Fujiwara, K.; Morisaki, T.; Fukuhara, T.; Kitano, H.; Takeuchi, H. Lenvatinib for anaplastic thyroid cancer and lenvatinib-induced thyroid dysfunction. Eur. Thyroid J. 2018, 7, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nervo, A.; Gallo, M.; Sama, M.T.; Felicetti, F.; Alfano, M.; Migliore, E.; Marchisio, F.; Berardelli, R.; Arvat, E.; Piovesan, A. Lenvatinib in advanced radioiodine-refractory thyroid cancer: A snapshot of real-life clinical practice. Anticancer Res. 2018, 38, 1643–1649. [Google Scholar] [CrossRef]
- Sugino, K.; Nagahama, M.; Kitagawa, W.; Ohkuwa, K.; Uruno, T.; Matsuzu, K.; Suzuki, A.; Masaki, C.; Akaishi, J.; Hames, K.Y.; et al. Clinical factors related to the efficacy of tyrosine kinase inhibitor therapy in radioactive iodine refractory recurrent differentiated thyroid cancer patients. Endocr. J. 2018, 65, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, C.; Kiyota, N.; Imamura, Y.; Goto, H.; Suto, H.; Chayahara, N.; Toyoda, M.; Ito, Y.; Miya, A.; Miyauchi, A.; et al. Exploratory analysis of prognostic factors for lenvatinib in radioiodine-refractory differentiated thyroid cancer. Head Neck 2019, 41, 3023–3032. [Google Scholar] [CrossRef]
- Tahara, M.; Brose, M.S.; Wirth, L.J.; Suzuki, T.; Miyagishi, H.; Fujino, K.; Dutcus, C.E.; Gianoukakis, A. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur. J. Cancer 2019, 106, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Kiyota, N.; Yamazaki, T.; Chayahara, N.; Nakano, K.; Inagaki, L.; Toda, K.; Enokida, T.; Minami, H.; Imamura, Y.; et al. A phase ii study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019, 15, 717–726. [Google Scholar] [CrossRef] [Green Version]
- Wirth, L.J.; Tahara, M.; Robinson, B.; Francis, S.; Brose, M.S.; Habra, M.A.; Newbold, K.; Kiyota, N.; Dutcus, C.E.; Mathias, E.; et al. Treatment-emergent hypertension and efficacy in the phase 3 study of (e7080) lenvatinib in differentiated cancer of the thyroid (select). Cancer 2018, 124, 2365–2372. [Google Scholar] [CrossRef]
- Yamazaki, H.; Iwasaki, H.; Takasaki, H.; Suganuma, N.; Sakai, R.; Masudo, K.; Nakayama, H.; Rino, Y.; Masuda, M. Efficacy and tolerability of initial low-dose lenvatinib to treat differentiated thyroid cancer. Medicine 2019, 98, e14774. [Google Scholar] [CrossRef]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Nilubol, N.; Zhang, L.; Kebebew, E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid 2013, 23, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Toniato, A.; Boschin, I.; Casara, D.; Mazzarotto, R.; Rubello, D.; Pelizzo, M. Papillary thyroid carcinoma: Factors influencing recurrence and survival. Ann. Surg. Oncol. 2008, 15, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Ancker, O.V.; Wehland, M.; Bauer, J.; Infanger, M.; Grimm, D. The adverse effect of hypertension in the treatment of thyroid cancer with multi-kinase inhibitors. Int. J. Mol. Sci. 2017, 18, 625. [Google Scholar] [CrossRef] [Green Version]
- Belum, V.R.; Serna-Tamayo, C.; Wu, S.; Lacouture, M.E. Incidence and risk of hand-foot skin reaction with cabozantinib, a novel multikinase inhibitor: A meta-analysis. Clin. Exp. Dermatol. 2016, 41, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Bellmunt, J.; Eisen, T.; Fishman, M.; Quinn, D. Experience with sorafenib and adverse event management. Crit. Rev. Oncol. Hematol. 2011, 78, 24–32. [Google Scholar] [CrossRef]



| Reference | Type of Study | Drug + Starting Dose | Objective | Type of Cancer | Patient Characteristics | Efficacy Outcome | Country |
|---|---|---|---|---|---|---|---|
| Balmelli et al., 2018 [35] | Retrospective | Lenvatinib, 24 mg | Efficacy and toxicity | RAI-refractory, metastatic DTC | Patients: 13 Median age: 72 (range: 37–81) Female: N/A | PFS: 7.2 months (95% CI, 0.8–13.7) | Switzerland |
| Gianoukakis et al., 2018 [36] | Randomized, double-blind, post hoc analysis | Lenvatinib, 24 mg | Duration of survival in responders | RAI-refractory DTC. PTC, FTC, HCC, and PDTC | Patients: 157 Female: 53.5% Age: ≤65: 66.2% Age >65: 33.8% | PFS: 33.1 months (95% CI, 27.8–44.6) | America, Europe, Asia, and Australia |
| Hu et al., 2019 [37] | Randomized, double-blind | Vandetanib, 150 mg, 300 mg | Efficacy and tolerability | Unresectable, locally advanced or metastatic MTC | Patients: 81 Female: 33.3% Mean age: 52.5 | 150 mg: RR: 20% (95% CI, 10.5%–34.8%) 300mg: RR: 29.3% (95% CI, 17.6%–44.5%) | Nine countries |
| Iwasaki et al., 2018 [33] | Retrospective | Lenvatinib, 24 mg, 20 mg, 14 mg, 10 mg | Safety and efficacy | ATC | Patients: 23 Female: 69.6% Median age: 77 (range: 42–89) | RR: 17.4% OS: 166 days | Japan |
| Iwasaki et al., 2019 [38] | Retrospective | Sorafenib, lenvatinib. Dose unknown | Efficacy | Metastatic PTC and FTC | Patients: 56 Female: 62.5% Median age: 70 (range 41–84) | RR: 28.5% | Japan |
| Jerkovich et al., 2019 [39] | Retrospective | Sorafenib | Efficacy and safety | PTC, FTC, and HCC | Patients: 18 Female: 54.6% a Median age: 61 (range 36–75) | Median PFS: 16.5 months | Argentina |
| Kim et al., 2018 [40] | Retrospective | Sorafenib Mean daily dose 666 ± 114 mg | Efficacy and safety | RAI-refractory DTC; PTC, FTC, HCC, and PDTC | Patients: 98 Female: 69% Median age: 65.6 (range 57.7–72.2) | PFS: 9.7 months (range 4.5–16.7) | Korea |
| Kim et al., 2019 [41] | Retrospective | Sorafenib Mean daily dose: 602 mg | Efficacy and safety | RAI-refractory locally advanced or metastatic DTC; PTC, FTC, and PDTC | Patients: 85 Female: 61% Median age: 55 (range 22–81) | Median PFS: 14.4 months (range 1.6–92.2) | Korea |
| Kim et al., 2019 [42] | Retrospective | Sorafenib ≤400 mg–800 mg Lenvatinib 20 mg | Safety | RAI-refractory locally advanced or metastatic DTC; PTC, FTC, HCC, and PDTC. | Patients Lenvatinib: 23 Female: 60.9% Median age: 59.7 (range 38.9–74.4) Patients Sorafenib: 48 Female: 58.3% Median age: 62.0 (range 32.6–79.0) | Not available (N/A) | Korea |
| Kocsis et al., 2018 [43] | Prospective | Sorafenib 400 mg × 2 | Efficacy and safety | Metastatic, progressive, or symptomatic MTC | Patients: 10 Female: 60% Mean age: 51.7 (range 25–71) | Median PFS: 19.1 months | Hungary |
| Koyama et al., 2018 [44] | Retrospective | Lenvatinib 24 mg | Efficacy and safety | ATC | Patients: 5 Female: 0% Mean age: 58.8 | Median OS: 165 days. RR: 60% | Japan |
| Locati et al., 2019 [32] | Retrospective | Lenvatinib 24 mg for 71% of patients | Efficacy and toxicity | RAI-refractory DTC | Patients: 94 Female: 48.9% Median age: 60 (range 23–82) | PFS: 10.8 months (95% CI, 7.7–12.6) | Italy |
| Molina-Vega et al., 2018 [34] | Retrospective | Sorafenib: 800 mg or 400 mg Lenvatinib: mean dose 21,6 mg | Efficacy and safety | RAI-refractory metastatic DTC; PTC, FTC, and HCC. | Patients Sorafenib: 16 Patients Lenvatinib: 1 Female: 47.1% Mean age: 64.7 | Median PFS: 18 months | Spain |
| Nervo et al., 2018 [45] | Retrospective | Lenvatinib 24 mg | Efficacy and safety | RAI-refractory DTC; PDTC, PTC, and FTC | Patients: 12 Female: 75% Median age: 61 (range 51.5–68) | PFS 6m: 63.6% (95% CI, 29.7–84.5)PFS 12m: 54.6% (95% CI, 22.9–78.0) | Italy |
| Sugino et al., 2018 [46] | Retrospective | Lenvatinib 24 mg | Efficacy | RAI-refractory DTC; PTC and FTC | Patients: 29 Female: 69% Median age: 66 (32–81) | Median PFS: 24.3 months | Japan |
| Suzuiki et al., 2019 [47] | Retrospective | Lenvatinib 24 mg | Prognostic and predictive factors | RAI-refractory DTC; PTC, FTC, PDTC | Patients: 26 Female: 69.2% Median age: 64 (range 30–83) | Two-year PFS: 58.4% | Japan |
| Tahara et al., 2019 [48] | Randomized double-blind, post hoc analysis | Lenvatinib 24 mg | Efficacy in dose-interrupted patients | Progressive, RAI-refractory PTC, PDTC, FTC, and HCC | Patients group 1b:134 Female: 49.3% Median age: 61.5 (range 27–83) Patients group 2c: 127 Female: 55.1% Median age: 65.0 (range 39–89) | Group 1:PFS: not reached (N/R)Group 2:PFS: 12.8 months (95% CI, 9.3–16.5) | America, Europe, Asia, and Australia |
| Takahashi et al., 2019 [49] | Nonrandomized phase II study | Lenvatinib 24 mg | Safety and efficacy | RAI-refractory DTC, MTC, and ATC. | Patients: 51 Female: 59% Median age: 61 (21–84) | PFS: RAI-Refractory DTC: 25.8 months (95% CI, 18.4–N/R)MTC: 9.2 months (95% CI, 1.8–N/R) ATC: 7.4 months (95% CI, 1.7–12.9) | Japan |
| Wirth et al., 2018 [50] | Randomized double-blind, post hoc analysis | Lenvatinib 24 mg | Efficacy and safety in patients with treatment-emergent hypertension | Progressive, RAI-refractory PTC, PDTC, FTC, and HCCd. | Patients: 190 Female: N/A Age: N/A | Median PFS: 18.8 months (95% CI, 16.5–N/R) | America, Europe, Asia, and Australia |
| Yamazaki et al., 2019 [51] | Retrospective | Lenvatinib 24, 20, 14, 10 mg | Compare low dose lenvatinib to full dose, 24 mg | DTC; PTC and FTC | Full dose: Patients: 30 Female: 67% Median age: 68 (range 47–83) Low dose: Patients: 6 Female: 83% Median age: 77 (range 41–84) | Full doseMedian PFS: 696 days (95% CI, 318–N/R) Low dose: Median PFS: N/R (95% CI, 124 days–N/R) | Japan |
| Reference | Drug + Starting Dose | Prevalence of AEs | AEs in ≥50% of Patients |
|---|---|---|---|
| Balmelli et al., 2018 [35] | Lenvatinib, 24 mg | 92% | Fatigue (50%) |
| Gianoukakis et al., 2018 [36] | Lenvatinib, 24 mg | 80.8%a | N/A |
| Hu et al., 2019 [37] | Vandetanib, 150 mg, 300 mg | 150 mg: 97.5% 300 mg: 97.6% | None ≥50% |
| Iwasaki et al., 2018 [33] | Lenvatinib, 24 mg, 20 mg | 100% | Hypertension (91%) Fatigue and anorexia (65%) Proteinuria (61%) |
| Iwasaki et al., 2019 [38] | Sorafenib, lenvatinib | N/A | N/A |
| Jerkovich et al., 2019 [39] | Sorafenib | 90% | Palmar-plantar erythrodysesthesia syndrome (67%) Diarrhea (52%) Hypertension (52%) |
| Kim et al., 2018 [40] | Sorafenib Mean daily dose 666 ± 114 mg | 95% | Palmar-plantar erythrodysesthesia syndrome (76%) |
| Kim et al., 2019 [41] | Sorafenib Mean daily dose: 602 mg | 64%b | N/A |
| Kim et al., 2019 [42] | Sorafenib: ≤400 mg–800 mg Lenvatinib: 20 mg | N/A | Lenvatinib: Palmar-plantar erythrodysesthesia syndrome (56.5%) Diarrhea (82.6%) Hypertension (78.3%) Decreased weight (52.2%) Sorafenib: Palmar-plantar erythrodysesthesia syndrome (87.5%) Diarrhea (62.5%) Anorexia (60.4%) Alopecia (56.3%) Mucositis (52.1%) Generalized weakness (50%) |
| Kocsis et al., 2018 [43] | Sorafenib 400 mg × 2 | 100% | Fatigue (60%) Palmar-plantar erythrodysesthesia syndrome (50%) Rash/dermatitis (50%) |
| Koyama et al., 2018 [44] | Lenvatinib 24 mg | 100% | Proteinuria (100%) Hypothyroidism (80%) Hypertension (80%) Fatigue (80%) Anorexia (80%) Decreased weight (80%) |
| Locati et al., 2019 [32] | Lenvatinib 24 mg for 71% of patients | 87.2% | N/A |
| Molina-Vega et al., 2018 [34] | Sorafenib: 800 mg or 400 mg Lenvatinib: mean dose 21.6 mg | 100% | Sorafenib: Fatigue (68.7%) Palmar-plantar erythrodysesthesia syndrome (68.7%) Diarrhea (62.5%) Lenvatinib: Fatigue (100%) Hypertension (80%) Palmar-plantar erythrodysesthesia syndrome (60%) Diarrhea (60%) |
| Nervo et al., 2018 [45] | Lenvatinib 24 mg | 100% | Decreased weight (91.7%) Palmar-plantar erythrodysesthesia syndrome (91.7%) Hypertension (75%) Nausea (75%) Diarrhea (66.7%) Fatigue (58.3%) Oral mucositis (58.3%) Decreased appetite (58.3%) Myalgia (58.3%) Arthralgia (50%) |
| Sugino et al., 2018 [46] | Lenvatinib 24 mg | 100% | Hypertension (75.9%) Palmar-plantar erythrodysesthesia syndrome (58.6%) |
| Suzuki et al., 2019 [47] | Lenvatinib 24 mg | 96.2%d | Proteinuria (61.5%)d Malaise (57.7%)d |
| Tahara et al., 2019 [48] | Lenvatinib 24 mg | Group 1e: 100% Group 2f: 99.2% | Group 1: Diarrhea (73.9%) Hypertension (69.4%) Decreased weight (56.7%) Group 2: Hypertension (69.3%) Decreased appetite (62.2%) Diarrhea (58.3%) |
| Takahashi et al., 2019 [49] | Lenvatinib 24 mg | 100% | RAI-refractory DTC: Hypertension (96%) Palmar-plantar erythrodysesthesia syndrome (92%) Fatigue (80%) Decreased appetite (68%) Stomatitis (68%) Proteinuria (60%) Diarrhea (60%) Arthralgia (56%) MTC: Decreased appetite (100%) Hypertension (89%) Palmar-plantar erythrodysesthesia syndrome (89%) Diarrhea (89%) Fatigue (78%) Proteinuria (67%) Insomnia (56%) ATC: Hypertension (82%) Decreased appetite (82%) Fatigue (59%) Proteinuria (59%) Nausea (59%) Insomnia (56%) |
| Wirth et al., 2018 [50] | Lenvatinib 24 mg | 100%g | Hypertension (100%)g |
| Yamazaki et al., 2019 [51] | Lenvatinib 24, 20, 14, 10 mg | Full dose: Unknown Low dose: 100% | Full dose: Hypertension (93%) Proteinuria (77%) Palmar-plantar erythrodysesthesia syndrome (77%) Low dose: Hypertension (100%) Proteinuria (83%) Palmar-plantar erythrodysesthesia syndrome (67%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ancker, O.V.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. Multikinase Inhibitor Treatment in Thyroid Cancer. Int. J. Mol. Sci. 2020, 21, 10. https://doi.org/10.3390/ijms21010010
Ancker OV, Krüger M, Wehland M, Infanger M, Grimm D. Multikinase Inhibitor Treatment in Thyroid Cancer. International Journal of Molecular Sciences. 2020; 21(1):10. https://doi.org/10.3390/ijms21010010
Chicago/Turabian StyleAncker, Ole Vincent, Marcus Krüger, Markus Wehland, Manfred Infanger, and Daniela Grimm. 2020. "Multikinase Inhibitor Treatment in Thyroid Cancer" International Journal of Molecular Sciences 21, no. 1: 10. https://doi.org/10.3390/ijms21010010
APA StyleAncker, O. V., Krüger, M., Wehland, M., Infanger, M., & Grimm, D. (2020). Multikinase Inhibitor Treatment in Thyroid Cancer. International Journal of Molecular Sciences, 21(1), 10. https://doi.org/10.3390/ijms21010010