The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum
Abstract
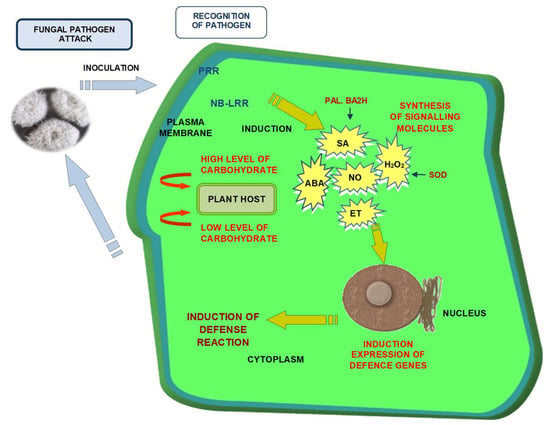
:1. Introduction
2. Results
2.1. Involvement of Soluble Sugars in the Regulation of the Level of Signaling Molecules as Well as in the Enzyme Activity in Embryo Axes of L. luteus L. cv. “Juno” in Response to F. oxysporum
2.1.1. Levels of Salicylic Acid (SA)
2.1.2. Levels of Abscisic Acid (ABA)
2.1.3. Levels of Ethylene Secretion (ET)
2.1.4. Hydrogen Peroxide (H2O2) Concentration
2.1.5. Phenylalanine Ammonia-Lyase (PAL) Activity
2.1.6. Benzoic Acid 2-Hydroxylase (BA2H) Activity
2.1.7. Superoxide Dismutase (SOD) Activity
2.1.8. Analysis of Disease Symptoms
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Spore Suspension and Inoculation
4.3. Biochemical Assays
4.3.1. Determination of Salicylic Acid (SA)
4.3.2. Determination of Abscisic Acid (ABA)
4.3.3. Determination of Ethylene (ET)
4.3.4. Determination of Hydrogen Peroxide Concentration
4.3.5. Phenylalanine Ammonia-Lyase (PAL) Assay
4.3.6. Benzoic Acid 2-Hydroxylase (BA2H) Assay
4.3.7. Superoxide Dismutase (SOD) Assay
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| +Sn | embryo axes noninoculated and cultured in vitro on Heller’s medium with 60 mM sucrose |
| +Gn | embryo axes noninoculated and cultured in vitro on medium with 120 mM glucose |
| +Fn | embryo axes noninoculated and cultured in vitro on medium with 120 mM fructose |
| −Sn | noninoculated cultured in vitro on medium without sucrose |
| +Si | inoculated and cultured with 60 mM sucrose |
| +Gi | inoculated and cultured with 120 mM glucose |
| +Fi | inoculated and cultured with 120 mM fructose |
| −Si | inoculated and cultured without sucrose |
| SA | salicylic acid |
| TSA | total salicylic acid |
| SAG | glucoside salicylic acid |
| ABA | abscisic acid |
| ET | ethylene |
| PAL | phenylalanine ammonia-lyase |
| SOD | superoxide dismutase |
| BA2H | benzoic acid 2-hydroxylase |
References
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdá, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morkunas, I.; Bednarski, W. Fusarium oxysporum-induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different sugar levels. J. Plant Physiol. 2008, 165, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Ratajczak, L. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol. Plant. 2014, 36, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Formela, M.; Samardakiewicz, S.; Marczak, Ł.; Nowak, W.; Narożna, D.; Bednarski, W.; Kasprowicz-Maluśki, A.; Morkunas, I. Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton. Molecules 2014, 19, 13392–13421. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Marczak, Ł.; Stachowiak, J.; Stobiecki, M. Sucrose-induced lupine defense against Fusarium oxysporum: Sucrose-stimulated accumulation of isoflavonoids as a defense response of lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2005, 43, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Gmerek, J. The possible involvement of peroxidase in defense of yellow lupine embryo axes against Fusarium oxysporum. J. Plant Physiol. 2007, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Kozłowska, M.; Ratajczak, L.; Marczak, Ł. Role of sucrose in the development of Fusarium wilt in lupine embryo axes. Physiol. Mol. Plant Pathol. 2007, 70, 25–37. [Google Scholar] [CrossRef]
- Morkunas, I.; Bednarski, W.; Kopyra, M. Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 2008, 72, 167–178. [Google Scholar] [CrossRef]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Govind, S.R.; Jogaiah, S.; Abdelrahman, M.; Shetty, H.S.; Tran, L.-S.P. Exogenous trehalose treatment enhances the activities of defense-related enzymes and triggers resistance against downy mildew disease of pearl millet. Front. Plant Sci. 2016, 7, 1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, aaf6395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roitsch, T. Source-sink regulation by sugar and stress. Curr. Opin. Plant Biol. 1999, 2, 198–206. [Google Scholar] [CrossRef]
- León, P.; Sheen, J. Sugar and hormone connections. Trends Plant Sci. 2003, 8, 110–116. [Google Scholar] [CrossRef]
- Rolland, F.; Sheen, J. Sugar sensing and signalling networks in plants. Biochem. Soc. Trans. 2005, 33, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Čeidaitė, A.; Sirtautas, R.; Duchovskis, P.; Kviklys, D. Effect of crop load on phytohormones, sugars, and biennial bearing in apple trees. Biol. Plant. 2016, 60, 394–400. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Herbers, K.; Meuwly, P.; Métraux, J.P.; Sonnewald, U. Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett. 1996, 397, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Loreti, E.; Povero, G.; Novi, G.; Solfanelli, C.; Alpi, A.; Perata, P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008, 179, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Bogatek, R.; Côme, D.; Corbineau, F.; Ranjan, R.; Lewak, S. Jasmonic acid affects dormancy and sugar catabolism in germinating apple embryos. Plant Physiol. Biochem. 2002, 40, 167–173. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Van de Poel, B.; Höfte, M.; Van den Ende, W. Sweet immunity: Inulin boosts resistance of lettuce (Lactuca sativa) against grey mold (Botrytis cinerea) in an ethylene-dependent manner. Int. J. Mol. Sci. 2019, 20, 1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazan, K.; Lyons, R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerli, L.; Stein, M.; Lipka, V.; Schulze-Lefert, P.; Somerville, S. Host and non-host pathogens elicit different jasmonate/ethylene responses in Arabidopsis. Plant J. 2004, 40, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Lamb, C. Systemic immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Halim, V.A.; Eschen-Lippold, L.; Altmann, S.; Birschwilks, M.; Scheel, D.; Rosahl, S. Salicylic acid is important for basal defense of Solanum tuberosum against Phytophthora infestans. Mol. Plant. Microbe Interact. 2007, 20, 1346–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. 2007, 104, 18842–18847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, K.; Shinozaki, K. (Eds.) Signal Crosstalk in Plant Stress Responses; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Derksen, H.; Rampitsch, C.; Daayf, F. Signaling cross-talk in plant disease resistance. Plant Sci. 2013, 207, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shah, J.; Klessig, D.F. Signal perception and transduction in plant defense responses. Genes Dev. 1997, 11, 1621–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheel, D. Resistance response physiology and signal transduction. Curr. Opin. Plant Biol. 1998, 1, 305–310. [Google Scholar] [CrossRef]
- Hidalgo, P.; Garretón, V.; Berríos, C.G.; Ojeda, H.; Jordana, X.; Holuigue, L. A nuclear casein kinase 2 activity is involved in early events of transcriptional activation induced by salicylic acid in tobacco. Plant Physiol. 2001, 125, 396–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant Biol. 1998, 1, 316–323. [Google Scholar] [CrossRef]
- Reymond, P.; Farmer, E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Rojo, E.; Solano, R.; Sánchez-Serrano, J.J. Interactions between signaling compounds involved in plant defense. J. Plant Growth Regul. 2003, 22, 82–98. [Google Scholar] [CrossRef]
- Riet, K.B.; Ndlovu, N.; Piater, L.A.; Dubery, I.A. Simultaneous analysis of defense-related phytohormones in Arabidopsis thaliana responding to fungal infection. Appl. Plant Sci. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Ehness, R.; Ecker, M.; Godt, D.E.; Roitsch, T. Glucose and stress independently regulate source and sink metabolism and defense mechanisms via signal transduction pathways involving protein phosphorylation. Plant Cell 1997, 9, 1825–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef] [PubMed]
- Khorsgani, O.A.; Flores, F.B.; Pessarakli, M. Plant signaling pathways involved in stomatal movement under drought stress conditions. Adv. Plants Agric. Res. 2018, 8, 290–297. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W. Sugars take a central position in plant growth, development and, stress responses. A focus on apical dominance. Front. Plant Sci. 2014, 5, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enyedi, A.J.; Raskin, I. Induction of UDP-glucose:salicylic acid glucosyltransferase activity in tobacco mosaic virus-inoculated tobacco (Nicotiana tabacum) leaves. Plant Physiol. 1993, 101, 1375–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faize, M.; Faize, L.; Koike, N.; Ishizaka, M.; Ishii, H. Acibenzolar-s-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology 2004, 94, 604–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Welti, R.; Wang, X. Simultaneous quantification of major phytohormones and related compounds in crude plant extracts by liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 2008, 69, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Grellet-Bournonville, C.F.; Martinez-Zamora, M.G.; Castagnaro, A.P.; Díaz-Ricci, J.C. Temporal accumulation of salicylic acid activates the defense response against Colletotrichum in strawberry. Plant Physiol. Biochem. 2012, 54, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Weihmann, T.; Li, X. Plant innate immunity. In Signaling and Communication in Plants: Plant-Environment Interactions; Baluska, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 119–136. [Google Scholar]
- Ding, L.; Xu, H.; Yi, H.; Yang, L.; Kong, Z.; Zhang, L.; Xue, S.; Jia, H.; Ma, Z. Resistance to hemi-biotrophic Fusarium graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS ONE 2011, 6, e19008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audenaert, K.; Pattery, T.; Cornelis, P.; Höfte, M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant Microbe Interact. 2002, 15, 1147–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohr, P.G.; Cahill, D.M. Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct. Plant Biol. 2003, 30, 461. [Google Scholar] [CrossRef]
- Thaler, J.S.; Bostock, R.M. Interactions between abscisic-acid-mediated responses and plant resistance to pathogens and insects. Ecology 2004, 85, 48–58. [Google Scholar] [CrossRef]
- Koga, H.; Dohi, K.; Mori, M. Abscisic acid and low temperatures suppress the whole plant-specific resistance reaction of rice plants to the infection of Magnaporthe grisea. Physiol. Mol. Plant Pathol. 2004, 65, 3–9. [Google Scholar] [CrossRef]
- Kunkel, B.N.; Brooks, D.M. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Navarro, L.; Bari, R.; Jones, J.D.G. Pathological hormone imbalances. Curr. Opin. Plant Biol. 2007, 10, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-H.; Yoo, S.-D. Signaling role of fructose mediated by FINS1/FBP in Arabidopsis thaliana. PLoS Genet. 2011, 7, e1001263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vleesschauwer, D.; Yang, Y.; Cruz, C.V.; Höfte, M. Abscisic acid-induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase-mediated repression of ethylene signaling. Plant Physiol. 2010, 152, 2036–2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.; Ma, X.; Tan, H.; Zhou, J. Abscisic acid enhances resistance to Alternaria solani in tomato seedlings. Plant Physiol. Biochem. 2011, 49, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Pflugmacher, M.; Klages, S.; Mäser, A.; Mock, A.; Stahl, D.J. Accumulation of the hormone abscisic acid (ABA) at the infection site of the fungus Cercospora beticola supports the role of ABA as a repressor of plant defence in sugar beet. Mol. Plant Pathol. 2008, 9, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.Y.; Yoshioka, K.; Desveaux, D. The roles of ABA in plant–pathogen interactions. J. Plant Res. 2011, 124, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jang, J.; Jones, T.L.; Sheen, J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 1998, 95, 10294–10299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genoud, T.; Métraux, J.-P. Crosstalk in plant cell signaling: Structure and function of the genetic network. Trends Plant Sci. 1999, 4, 503–507. [Google Scholar] [CrossRef]
- Sheen, J.; Zhou, L.; Jang, J.C. Sugars as signaling molecules. Curr. Opin. Plant Biol. 1999, 2, 410–418. [Google Scholar] [CrossRef]
- Hückelhoven, R.; Fodor, J.; Preis, C.; Kogel, K.-H. Hypersensitive cell death and papilla formation in barley attacked by the powdery mildew fungus are associated with hydrogen peroxide but not with salicylic acid accumulation. Plant Physiol. 1999, 119, 1251–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Able, A.J. Role of reactive oxygen species in the response of barley to necrotrophic pathogens. Protoplasma 2003, 221, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Lubaina, A.S.; Murugan, K. Biochemical Characterization of Oxidative Burst during Interaction between Sesame (Sesamum indicum L.) in Response to Alternaria sesami. In Prospects in Bioscience: Addressing the Issues; Sabu, A., Augustine, A., Eds.; Springer: New Delhi, India, 2013; pp. 243–250. [Google Scholar]
- Shetty, N.P.; Kristensen, B.K.; Newman, M.-A.; Møller, K.; Gregersen, P.L.; Jørgensen, H.J.L. Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol. Mol. Plant Pathol. 2003, 62, 333–346. [Google Scholar] [CrossRef]
- Heller, R. Recherches sur la nutrition minerale des tissues vegetaux cultives in vitro. Ann. Sci. Nat. Bot. Biol. Veg. 1954, 14, 1–223. [Google Scholar]
- Yalpani, N.; Leon, J.; Lawton, M.A.; Raskin, I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993, 103, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R. Abscisic acid is not necessary for gravitropism in primary roots of Zea mays. Ann. Bot. 1990, 66, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Stroiński, A. ABA and proline accumulation in leaves and roots of wild (Hordeum spontaneum) and cultivated (Hordeum vulgare ‘Maresi’) barley genotypes under water deficit conditions. Acta Physiol. Plant. 2003, 25, 55–61. [Google Scholar] [CrossRef]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221–222, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Aparicio-Tejo, P.; Irigoyen, J.J.; Sanchez-Diaz, M. Some enzymes of hydrogen peroxide metabolism in leaves and root nodules of Medicago sativa. Plant Physiol. 1986, 82, 1169–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahill, D.M.; McComb, J.A. A comparison of changes in phenylalanine ammonia-lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calophylla (field resistant) and E. marginata (susceptible) when infected with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 1992, 40, 315–332. [Google Scholar] [CrossRef]
- Leon, J.; Shulaev, V.; Yalpani, N.; Lawton, M.A.; Raskin, I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc. Natl. Acad. Sci. USA 1995, 92, 10413–10417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]









| Culture Variant | Disease Symptoms | |
|---|---|---|
| 72 h | 96 h | |
| + Sn | None | None |
| + Si | Necrotic changes mainly at the vertices of the shoot axes near the site of inoculation, visible brown discoloration on the roots, slow loss of turgor | Progressing necrosis on the entire surface of the embryo axes, some of the axes without turgor |
| + Gn | None | None |
| + Gi | Necrotic changes mainly at the vertices of the shoot axes near the site of inoculation, visible brown discoloration on the roots, slightly thicker and longer roots and weaker symptoms in relation to Si + and + Fi, slow loss of turgor | Progressing necrosis on the entire surface of the embryo axes, some of the axes without of turgor, less necrosis compared to + Si and + Fi |
| + Fn | None | None |
| + Fi | Necrotic changes mainly at the vertices of the shoot axes near the site of inoculation, visible brown discoloration on the roots, slow loss of turgor | Progressing necrosis on the entire surface of the embryo axes, some of the axes without turgor |
| −Sn | None | none |
| −Si | Koss of turgor, axes quite substantial overgrow with mycelium, white spots, inhibited elongation growth, brown discoloration almost whole axes, single axes completely white | Significant loss of turgor, axes strongly overgrown with mycelium, necrosis and brown discoloration of whole axes, dieback whole axes, lividity tissues |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formela-Luboińska, M.; Chadzinikolau, T.; Drzewiecka, K.; Jeleń, H.; Bocianowski, J.; Kęsy, J.; Labudda, M.; Jeandet, P.; Morkunas, I. The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. Int. J. Mol. Sci. 2020, 21, 4133. https://doi.org/10.3390/ijms21114133
Formela-Luboińska M, Chadzinikolau T, Drzewiecka K, Jeleń H, Bocianowski J, Kęsy J, Labudda M, Jeandet P, Morkunas I. The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. International Journal of Molecular Sciences. 2020; 21(11):4133. https://doi.org/10.3390/ijms21114133
Chicago/Turabian StyleFormela-Luboińska, Magda, Tamara Chadzinikolau, Kinga Drzewiecka, Henryk Jeleń, Jan Bocianowski, Jacek Kęsy, Mateusz Labudda, Philippe Jeandet, and Iwona Morkunas. 2020. "The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum" International Journal of Molecular Sciences 21, no. 11: 4133. https://doi.org/10.3390/ijms21114133
APA StyleFormela-Luboińska, M., Chadzinikolau, T., Drzewiecka, K., Jeleń, H., Bocianowski, J., Kęsy, J., Labudda, M., Jeandet, P., & Morkunas, I. (2020). The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. International Journal of Molecular Sciences, 21(11), 4133. https://doi.org/10.3390/ijms21114133