Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Sponges
2.2. Hematoxylin–Eosin Staining for Bone Defects
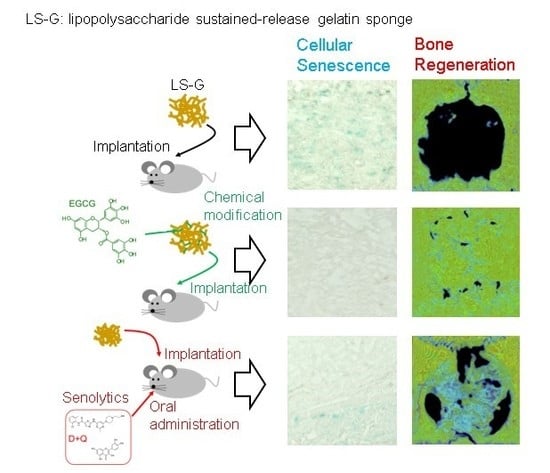
2.3. Increase in SIPS Cell after LS-G Implantation
2.4. Histomorphometric Analysis of Newly Formed Bone
2.5. Oxidation in the Bone Defects
3. Discussion
4. Materials and Methods
4.1. Characterization of the Sponges
4.2. Cytotoxic Effect of the Sponges
4.3. Animal Experiments
4.4. Histomorphometric Analysis
4.5. Histological and Immunofluorescent Staining
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LPS | lipopolysaccharide |
| LS-G | LPS sustained-release gelatin sponge |
| EGCG | epigallocatechin gallate |
| D+Q | dasatinib and quercetin |
| CBSD | critical-sized bone defect |
| OA | oral administration |
References
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus 2010, 29, E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Fridlyanskaya, I.; Alekseenko, L.; Nikolsky, N. Senescence as a general cellular response to stress: A mini-review. Exp. Gerontol. 2015, 72, 124–128. [Google Scholar] [CrossRef]
- Gorbunova, V.; Seluanov, A.; Pereira-Smith, O.M. Expression of Human Telomerase (hTERT) Does Not Prevent Stress-induced Senescence in Normal Human Fibroblasts but Protects the Cells from Stress-induced Apoptosis and Necrosis. J. Biol. Chem. 2002, 277, 38540–38549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Despars, G.; Carbonneau, C.L.; Bardeau, P.; Coutu, D.; Beauséjour, C. Loss of the Osteogenic Differentiation Potential during Senescence Is Limited to Bone Progenitor Cells and Is Dependent on p53. PLoS ONE 2013, 8, e73206. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Nourazarian, A.; Kangari, P.; Salmaninejad, A. Roles of Oxidative Stress in the Development and Progression of Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ben-Porath, I.; Weinberg, R.A. When cells get stressed: An integrative view of cellular senescence. J. Clin. Investig. 2004, 113, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, J.N.; Khosla, S. Cellular senescence in bone. Bone 2019, 121, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Endotoxin: The uninvited guest. Biomaterials 2005, 26, 6811–6817. [Google Scholar] [CrossRef] [PubMed]
- Reikerås, O.; Shegarfi, H.; Wang, J.E.; Utvåg, S.E. Lipopolysaccharide impairs fracture healing: An experimental study in rats. Acta Orthop. 2005, 76, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.K.; Jaiswal, M.K.; Agrawal, V.; Chaturvedi, M.M. Bacterial endotoxin (LPS)—Induced DNA damage in preimplanting embryonic and uterine cells inhibits implantation. Fertil. Steril. 2009, 91, 2095–2103. [Google Scholar] [CrossRef]
- Kim, C.O.; Huh, A.J.; Han, S.H.; Kim, J.M. Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch. Gerontol. Geriatr. 2012, 54, e35–e41. [Google Scholar] [CrossRef]
- Feng, X.; Feng, G.; Xing, J.; Shen, B.; Tan, W.; Huang, D.; Lu, X.; Tao, T.; Zhang, J.; Li, L.; et al. Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res. 2014, 356, 369–380. [Google Scholar] [CrossRef]
- Zhao, J.; Honda, Y.; Tanaka, T.; Hashimoto, Y.; Matsumoto, N. Releasing Behavior of Lipopolysaccharide from Gelatin Modulates Inflammation, Cellular Senescence, and Bone Formation in Critical-Sized Bone Defects in Rat Calvaria. Materials 2019, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Farr, J.N.; Xu, M.; Weivoda, M.M.; Monroe, D.G.; Fraser, D.G.; Onken, J.L.; Negley, B.A.; Sfeir, J.; Ogrodnik, M.; Hachfeld, C.M.; et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017, 23, 1072–1079. [Google Scholar] [CrossRef]
- DeMaria, M.; Ohtani, N.; Youssef, S.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- White, R.R.; Vijg, J. Do DNA double-strand breaks drive aging? Mol. Cell 2016, 63, 729–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.-L.; Lu, Z.-Y.; Zhang, X.; Yao, G.-D.; Liu, X.-L.; Wu, Q.-J.; Hayashi, T.; Yamato, M.; Fujisaki, H.; Hattori, S.; et al. Gelatin promotes cell aggregation and pro-inflammatory cytokine production in PMA-stimulated U937 cells by augmenting endocytosis-autophagy pathway. Int. J. Biochem. Cell Biol. 2018, 95, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. Part A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Shibayama, Y.; Asaka, S.; Nakata, K. Endotoxin hepatotoxicity augmented by ethanol. Exp. Mol. Pathol. 1991, 55, 196–202. [Google Scholar] [CrossRef]
- Hoban, D.B.; Connaughton, E.; Connaughton, C.; Hogan, G.; Thornton, C.; Mulcahy, P.; Moloney, T.C.; Dowd, E. Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013, 27, 91–100. [Google Scholar] [CrossRef]
- Croes, M.; Kruyt, M.; Loozen, L.; Kragten, A.; Yuan, H.; Dhert, W.; Öner, F.; Alblas, J. Local induction of inflammation affects bone formation. Eur. Cells Mater. 2017, 33, 211–226. [Google Scholar] [CrossRef]
- Strålberg, F.; Kassem, A.; Kasprzykowski, F.; Abrahamson, M.; Grubb, A.; Lindholm, C.; Lerner, U.H. Inhibition of lipopolysaccharide-induced osteoclast formation and bone resorption in vitro and in vivo by cysteine proteinase inhibitors. J. Leukoc. Biol. 2017, 101, 1233–1243. [Google Scholar] [CrossRef]
- Peairs, A.; Dai, R.; Gan, L.; Shimp, S.; Rylander, M.N.; Li, L.; Reilly, C. Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell. Mol. Immunol. 2010, 7, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Katsumata, Y.; Kanzaki, H.; Honda, Y.; Tanaka, T.; Yamaguchi, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; et al. Single Local Injection of Epigallocatechin Gallate-Modified Gelatin Attenuates Bone Resorption and Orthodontic Tooth Movement in Mice. Polymers 2018, 10, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.-H.; Jeon, H.-J.; Park, J.; Chang, M.-S. Epigallocatechin-3-gallate prevents oxidative stress-induced cellular senescence in human mesenchymal stem cells via Nrf2. Int. J. Mol. Med. 2016, 38, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Sharma, R.; Kumari, A.; Gulati, A.; Padwad, Y.S.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2019, 20, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local Controlled Release of Polyphenol Conjugated with Gelatin Facilitates Bone Formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, Y.; Huang, A.; Zhao, J.; Han, X.; Kurushima, Y.; Gong, Y.; Kanzaki, H.; Katsumata, Y.; Yamada, Y.; Hashimoto, Y.; et al. Sustained Release of Catechin from Gelatin and Its Effect on Bone Formation in Critical Sized Defects in Rat Calvaria. J. Hard Tissue Biol. 2020, 29, 77–84. [Google Scholar] [CrossRef]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; Van Deursen, J. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Palmer, A.K.; Xu, M.; Zhu, Y.; Pirtskhalava, T.; Weivoda, M.M.; Hachfeld, C.M.; Prata, L.G.; Van Dijk, T.H.; Verkade, E.; Casaclang-Verzosa, G.; et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 2019, 18, e12950. [Google Scholar] [CrossRef]
- Odgren, P.R.; Mackay, C.A.; Mason-Savas, A.; Yang, M.; Mailhot, G.; Birnbaum, M.J. False-Positive β-Galactosidase Staining in Osteoclasts by Endogenous Enzyme: Studies in Neonatal and Month-Old Wild-Type Mice. Connect. Tissue Res. 2006, 47, 229–234. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Paradoxes of senolytics. Aging 2018, 10, 4289–4293. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated (b)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 2000, 113, 3613–3622. [Google Scholar]
- Han, X.; Wu, Y.C.; Meng, M.; Sun, Q.S.; Gao, S.M.; Sun, H. Linarin prevents LPS induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NFkappaB pathways. Int. J. Mol. Med. 2018, 42, 1460–1472. [Google Scholar] [PubMed] [Green Version]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Takeda, Y.; Matsumoto, N.; Hashimoto, Y.; Baba, S.; Tanaka, T.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; et al. Epigallocatechin Gallate-Modified Gelatin Sponges Treated by Vacuum Heating as a Novel Scaffold for Bone Tissue Engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campisi, J. Senescent Cells, Tumor Suppression, and Organismal Aging: Good Citizens, Bad Neighbors. Cell 2005, 120, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Hara, E.; Honda, Y.; Suzuki, O.; Tanaka, T.; Matsumoto, N. Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones. Int. J. Mol. Sci. 2018, 19, 3232. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, T.; Kawamoto, K. Preparation of Thin Frozen Sections from Nonfixed and Undecalcified Hard Tissues Using Kawamot’s Film Method (2012). In Skeletal Development and Repair; Humana Press: Totowa, NJ, USA, 2014; Volume 1130, pp. 149–164. [Google Scholar]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, Y.; Huang, A.; Tanaka, T.; Han, X.; Gao, B.; Liu, H.; Wang, X.; Zhao, J.; Hashimoto, Y.; Yamamoto, K.; et al. Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics. Int. J. Mol. Sci. 2020, 21, 4213. https://doi.org/10.3390/ijms21124213
Honda Y, Huang A, Tanaka T, Han X, Gao B, Liu H, Wang X, Zhao J, Hashimoto Y, Yamamoto K, et al. Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics. International Journal of Molecular Sciences. 2020; 21(12):4213. https://doi.org/10.3390/ijms21124213
Chicago/Turabian StyleHonda, Yoshitomo, Anqi Huang, Tomonari Tanaka, Xiaoyu Han, Beiyuan Gao, Haitao Liu, Xinchen Wang, Jianxin Zhao, Yoshiya Hashimoto, Kazuyo Yamamoto, and et al. 2020. "Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics" International Journal of Molecular Sciences 21, no. 12: 4213. https://doi.org/10.3390/ijms21124213
APA StyleHonda, Y., Huang, A., Tanaka, T., Han, X., Gao, B., Liu, H., Wang, X., Zhao, J., Hashimoto, Y., Yamamoto, K., Matsumoto, N., Baba, S., & Umeda, M. (2020). Augmentation of Bone Regeneration by Depletion of Stress-Induced Senescent Cells Using Catechin and Senolytics. International Journal of Molecular Sciences, 21(12), 4213. https://doi.org/10.3390/ijms21124213