Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-Like” Behavioral Study with Proposed Analgesic Pathways
Abstract
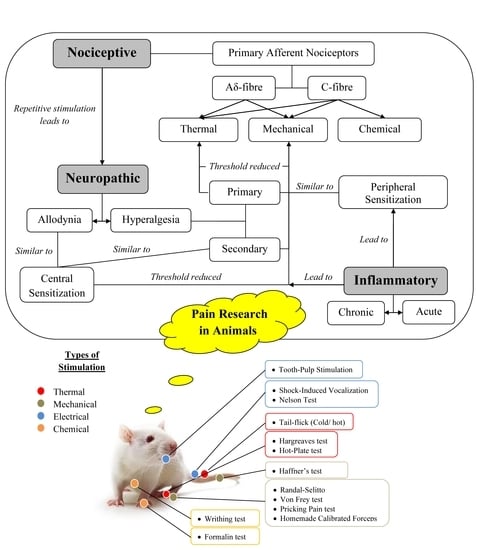
:1. Introduction
2. Non-Inflammatory Behavioral Pain Study in Animal Models
2.1. Phasic Pain
2.1.1. Thermal Stimuli
- i.
- Tail-Flick Test
- ii.
- Hot-Plate Test
- iii.
- Paw Withdrawal/Hargreaves Test
- iv.
- Cold Stimuli
2.1.2. Mechanical Stimuli
- i.
- Randall–SelittoTest
- ii.
- Pricking Test
- iii.
- Haffner’s/Tail-Pinch Method
- iv.
- Homemade Calibrated Forceps
- v.
- Von Frey Filament
2.1.3. Electrical Stimuli
- i.
- Shock-Induced Vocalization Test
- ii.
- Tail Shock Test/Nelson Test
- iii.
- Tooth-Pulp Stimulation Test
2.2. Tonic and Visceral Pain
Chemical Stimuli
- i.
- Formalin Test
- ii.
- Writhing Test
3. Inflammatory Pain Study in Animal Models
3.1. Complete Freund’s Adjuvant
3.2. Carrageenan Model
3.3. Formalin Model
3.4. Zymosan and Mustard Oil Models
3.5. Capsaicin Model
3.6. Bee Venom Model
4. Assessment of Arthritis and Inflammatory Pain in Animal Models
4.1. Weight Bearing
4.2. Gait and Posture Analysis
4.3. Spontaneous Mobility
4.4. Thermal and Mechanical Sensitivity of Paws
4.5. Mechanical Sensitivity Test
4.6. Struggle Threshold Angle
4.7. Vocalization Threshold
4.8. Plethysmometer and Micrometer Measurements
4.9. Grimace Scale Test
5. Limitations of Present Study
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinke, C.; Schmidt, K.; Forkmann, K.; Bingel, U. Phasic and tonic pain differentially impact the interruptive function of pain. PLoS ONE 2015, 10, e0118363. [Google Scholar] [CrossRef]
- Chang, C.; Shyu, B.C. A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain Res. 2001, 897, 71–81. [Google Scholar] [CrossRef]
- Rainville, P. Brain mechanisms of pain affect and pain modulation. Curr. Opin. Neurobiol. 2002, 12, 195–204. [Google Scholar] [CrossRef]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milind, P.; Monu, Y. Laboratory models for screening analgesics. Int Res.J. Pharm. 2013, 4, 15–19. [Google Scholar]
- Chu, L.F.; Angst, M.S.; Clark, D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin. J. Pain 2008, 24, 479–496. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.M. Animal Models of Pain; Humana Press: Totowa, NJ, USA, 2010. [Google Scholar]
- Mitra, S. Opioid-induced hyperalgesia: Pathophysiology and clinical implications. J. Opioid Manag. 2008, 4, 123–130. [Google Scholar] [CrossRef]
- Singh, V.P.; Jain, N.K.; Kulkarni, S.K. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001, 915, 218–226. [Google Scholar] [CrossRef]
- Scott, D.A.; Wright, C.E.; Angus, J.A. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain 2004, 109, 124–131. [Google Scholar] [CrossRef]
- Dyson, A.; Peacock, M.; Chen, A.; Courade, J.P.; Yaqoob, M.; Groarke, A.; Brain, C.; Loong, Y.; Fox, A. Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat. Pain 2005, 116, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Hache, G.; Guiard, B.P.; Le Dantec, Y.; Orvoen, S.; David, D.J.; Gardier, A.M.; Coudore, F. Antinociceptive effects of fluoxetine in a mouse model of anxiety/depression. Neuroreport 2012, 23, 525–529. [Google Scholar] [CrossRef]
- Aziz, M.A. Qualitative phytochemical screening and evaluation of anti-inflammatory, analgesic and antipyretic activities of Microcos paniculata barks and fruits. J. Integr. Med. 2015, 13, 173–184. [Google Scholar] [CrossRef]
- Wolinska, R.; Lesniak, A.; Zochowska, M.; Sacharczuk, M.; Kiec-Kononowicz, K.; Bujalska-Zadrozny, M. Antinociceptive effect of co-administered NMDA and histamine H4 receptor antagonists in a rat model of acute pain. Pharmacol. Rep. 2017, 69, 222–228. [Google Scholar] [CrossRef]
- Yemitan, O.K.; Adeyemi, O.O. Mechanistic assessment of the analgesic, anti-inflammatory and antipyretic actions of Dalbergia saxatilis in animal models. Pharm. Biol. 2017, 55, 898–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, A.; Hamdy, M.M.; Elbadr, M.M. Uses of fluoxetine in nociceptive pain management: A literature overview. Eur. J. Pharmacol. 2018, 829, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, M.; Iba, K.; Dohke, T.; Kanaya, K.; Okazaki, S.; Yamashita, T. Antagonists to TRPV1, ASICs and P2X have a potential role to prevent the triggering of regional bone metabolic disorder and pain-like behavior in tail-suspended mice. Bone 2018, 110, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Towett, P.K.; Kanui, T.I. Effects of pethidine, acetylsalicylic acid, and indomethacin on pain and behavior in the mole-rat. Pharmacol. Biochem. Behav. 1993, 45, 153–159. [Google Scholar] [CrossRef]
- Schreiber, S.; Pick, C.G. From selective to highly selective SSRIs: A comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur. Neuropsychopharmacol. 2006, 16, 464–468. [Google Scholar] [CrossRef]
- Patil, B.V.; Binjawadgi, A.; Kakkeri, R.; Raikar, S.; Anandi, B. A comparative study of analgesic activity of fluoxetine with ibuprofen and pentazocine in rodent models. J. Evol. Med. Dent. Sci. 2013, 2, 6261–6270. [Google Scholar]
- Manjunatha, C.; Ratnakar, J.S. A comparative experimental study of antinociceptive activity of fluoxetine with pentazocine in rodent models. Pharm. Innov. 2015, 4, 43. [Google Scholar]
- Masocha, W.; Thomas, A. Indomethacin plus minocycline coadministration relieves chemotherapy and antiretroviral drug-induced neuropathic pain in a cannabinoid receptors-dependent manner. J. Pharmacol. Sci. 2019, 139, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Rhodes, J.S.; Girard, I.; Gammie, S.C.; Garland, T., Jr. Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiol. Behav. 2004, 83, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.C.; Huang, C.C.; Hsu, K.S. The synthetic cannabinoids attenuate allodynia and hyperalgesia in a rat model of trigeminal neuropathic pain. Neuropharmacology 2007, 53, 169–177. [Google Scholar] [CrossRef]
- Liu, T.; Bai, Z.T.; Pang, X.Y.; Chai, Z.F.; Jiang, F.; Ji, Y.H. Degranulation of mast cells and histamine release involved in rat pain-related behaviors and edema induced by scorpion Buthus martensi Karch venom. Eur. J. Pharmacol. 2007, 575, 46–56. [Google Scholar] [CrossRef]
- Naono, R.; Nakayama, T.; Ikeda, T.; Matsushima, O.; Nishimori, T. Leucine at the carboxyl-terminal of endokinins C and D contributes to elicitation of the antagonistic effect on substance P in rat pain processing. Brain Res. 2007, 1165, 71–80. [Google Scholar] [CrossRef]
- Martucci, C.; Trovato, A.E.; Costa, B.; Borsani, E.; Franchi, S.; Magnaghi, V.; Panerai, A.E.; Rodella, L.F.; Valsecchi, A.E.; Sacerdote, P.; et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 2008, 137, 81–95. [Google Scholar] [CrossRef]
- Parenti, C.; Arico, G.; Ronsisvalle, G.; Scoto, G.M. Supraspinal injection of Substance P attenuates allodynia and hyperalgesia in a rat model of inflammatory pain. Peptides 2012, 34, 412–418. [Google Scholar] [CrossRef]
- Vera, G.; Cabezos, P.A.; Martin, M.I.; Abalo, R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol. Biochem. Behav. 2013, 105, 205–212. [Google Scholar] [CrossRef]
- Chen, Y.W.; Tzeng, J.I.; Lin, M.F.; Hung, C.H.; Wang, J.J. Forced treadmill running suppresses postincisional pain and inhibits upregulation of substance P and cytokines in rat dorsal root ganglion. J. Pain 2014, 15, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Khodorova, A.; Nicol, G.D.; Strichartz, G. The TrkA receptor mediates experimental thermal hyperalgesia produced by nerve growth factor: Modulation by the p75 neurotrophin receptor. Neuroscience 2017, 340, 384–397. [Google Scholar] [CrossRef] [Green Version]
- Kalinichev, M.; Donovan-Rodriguez, T.; Girard, F.; Haddouk, H.; Royer-Urios, I.; Schneider, M.; Bate, S.T.; Marker, C.; Pomonis, J.D.; Poli, S. ADX71943 and ADX71441, novel positive allosteric modulators of the GABAB receptor with distinct central/peripheral profiles, show efficacy in the monosodium iodoacetate model of chronic osteoarthritis pain in the rat. Eur. J. Pharmacol. 2017, 795, 43–49. [Google Scholar] [CrossRef]
- Kosiorek-Witek, A.; Makulska-Nowak, H.E. Morphine Analgesia Modification in Normotensive and Hypertensive Female Rats after Repeated Fluoxetine Administration. Basic Clin. Pharmacol. Toxicol. 2016, 118, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Ozaki, N.; Taguchi, T.; Mizumura, K.; Furukawa, K.; Sugiura, Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain 2008, 140, 292–304. [Google Scholar] [CrossRef]
- Bianchi, M.; Franchi, S.; Ferrario, P.; Sotgiu, M.L.; Sacerdote, P. Effects of the bisphosphonate ibandronate on hyperalgesia, substance P, and cytokine levels in a rat model of persistent inflammatory pain. Eur. J. Pain 2008, 12, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Akunne, H.C.; Soliman, K.F. Serotonin modulation of pain responsiveness in the aged rat. Pharmacol. Biochem. Behav. 1994, 48, 411–416. [Google Scholar] [CrossRef]
- Moon, H.C.; Lee, Y.J.; Cho, C.B.; Park, Y.S. Suppressed GABAergic signaling in the zona incerta causes neuropathic pain in a thoracic hemisection spinal cord injury rat model. Neurosci. Lett. 2016, 632, 55–61. [Google Scholar] [CrossRef]
- Luongo, L.; de Novellis, V.; Gatta, L.; Palazzo, E.; Vita, D.; Guida, F.; Giordano, C.; Siniscalco, D.; Marabese, I.; De Chiaro, M.; et al. Role of metabotropic glutamate receptor 1 in the basolateral amygdala-driven prefrontal cortical deactivation in inflammatory pain in the rat. Neuropharmacology 2013, 66, 317–329. [Google Scholar] [CrossRef]
- Genari, B.; Ferreira, M.B.C.; Medeiros, L.F.; de Freitas, J.S.; Cioato, S.G.; da Silva Torres, I.L.; Pohlmann, A.R.; Guterres, S.S.; Leitune, V.C.B.; Collares, F.M. Anti-inflammatory effect of an adhesive resin containing indomethacin-loaded nanocapsules. Arch. Oral Biol. 2017, 84, 106–111. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Bouhana, K.S.; Trollinger, D.; Winkler, J.; Lee, P.; Andrews, S.W. Sustained blockade of neurotrophin receptors TrkA, TrkB and TrkC reduces non-malignant skeletal pain but not the maintenance of sensory and sympathetic nerve fibers. Bone 2011, 48, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salimi, S.; Tamaddonfard, E. Microinjection of histamine and its H3 receptor agonist and antagonist into the agranular insular cortex influence sensory and affective components of neuropathic pain in rats. Eur. J. Pharmacol. 2019, 857, 172450. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Puma, S.L.; Landini, L.; Tuccinardi, T.; Poli, G.; Preti, D.; De Siena, G.; Patacchini, R.; Tsagareli, M.G.; Geppetti, P. The acyl-glucuronide metabolite of ibuprofen has analgesic and anti-inflammatory effects via the TRPA1 channel. Pharmacol. Res. 2019, 142, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Saffarpour, S.; Shaabani, M.; Naghdi, N.; Farahmandfar, M.; Janzadeh, A.; Nasirinezhad, F. In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol. Behav. 2017, 175, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Srebro, D.; Vuckovic, S.; Prostran, M. Participation of peripheral TRPV1, TRPV4, TRPA1 and ASIC in a magnesium sulfate-induced local pain model in rat. Neuroscience 2016, 339, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Jang, I.-S. Indomethacin inhibits tetrodotoxin-resistant Na+ channels at acidic pH in rat nociceptive neurons. Neuropharmacology 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Kaushal, R.; Taylor, B.K.; Jamal, A.B.; Zhang, L.; Ma, F.; Donahue, R.; Westlund, K.N. GABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAalpha1 receptors in the medial prefrontal cortex. Neuroscience 2016, 334, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Grundy, D.; Brookes, S. Mechanosensory Transduction. Enclyclopedia of Neuroscience 2009, 697–702. [Google Scholar] [CrossRef]
- Pedersen, L.H.; Scheel-Krüger, J.; Blackburn-Munro, G. Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 2007, 127, 17–26. [Google Scholar] [CrossRef]
- Lee, S.E.; Kim, J.H. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci. Res. 2007, 58, 245–249. [Google Scholar] [CrossRef]
- Messing, R.B.; Phebus, L.; Fisher, L.A.; Lytle, L.D. Analgesic effect of fluoxetine hydrochloride (Lilly 110140), a specific inhibitor of serotonin uptake. Psychopharmacol. Commun. 1975, 1, 511–521. [Google Scholar] [PubMed]
- Abdel-Salam, O.M.; Nofal, S.M.; El-Shenawy, S.M. Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacol. Res. 2003, 48, 157–165. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vale, M.L.; Thomazzi, S.M.; Paschoalato, A.B.; Poole, S.; Ferreira, S.H.; Cunha, F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000, 387, 111–118. [Google Scholar] [CrossRef]
- Voilley, N. Acid-sensing ion channels (ASICs): New targets for the analgesic effects of non-steroid anti-inflammatory drugs (NSAIDs). Curr. Drug Targets Inflamm. Allergy 2004, 3, 71–79. [Google Scholar] [CrossRef]
- Naderi, N.; Shafaghi, B.; Khodayar, M.-J.; Zarindast, M.-R. Interaction between gamma-aminobutyric acid GABAB and cannabinoid CB1 receptors in spinal pain pathways in rat. Eur. J. Pharmacol. 2005, 514, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, P.; Bisogno, T.; Punwar, S.; Farquhar-Smith, W.P.; Ambrosino, G.; Di Marzo, V.; Rice, A.S. Role of the endogenous cannabinoid system in the formalin test of persistent pain in the rat. Eur. J. Pharmacol. 2000, 396, 85–92. [Google Scholar] [CrossRef]
- Gameiro, G.H.; Gameiro, P.H.; Andrade Ada, S.; Pereira, L.F.; Arthuri, M.T.; Marcondes, F.K.; Veiga, M.C. Nociception- and anxiety-like behavior in rats submitted to different periods of restraint stress. Physiol. Behav. 2006, 87, 643–649. [Google Scholar] [CrossRef]
- Zhao, Z.-Q.; Chiechio, S.; Sun, Y.-G.; Zhang, K.-H.; Zhao, C.-S.; Scott, M.; Johnson, R.L.; Deneris, E.S.; Renner, K.J.; Gereau, R.W. Mice lacking central serotonergic neurons show enhanced inflammatory pain and an impaired analgesic response to antidepressant drugs. J. Neurosci. 2007, 27, 6045–6053. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanzadeh, B.; Mansouri, M.T.; Naghizadeh, B.; Alboghobeish, S. Local antinociceptive action of fluoxetine in the rat formalin assay: Role of l-arginine/nitric oxide/cGMP/KATP channel pathway. Can. J. Physiol. Pharmacol. 2018, 96, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Simoes, R.R.; Kraus, S.I.; Rosso, R.; Bridi, A.; Casoti, R.; Dahmer, J.; Morel, A.F.; Dos Santos, A.R.S.; Zanchet, E.M. Root bark of Discaria americana attenuates pain: A pharmacological evidence of interaction with opioidergic system and TRP/ASIC channels. J. Ethnopharmacol. 2018, 227, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Brandão, M.L.; Graeff, F.G. Neurobiology of Mental Disorders; Nova Biomedical: Waltham, MA, USA, 2006. [Google Scholar]
- D′amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Taniguchi, T.; Fan, X.T.; Kitamura, K.; Oka, T. Effects of peptidase inhibitors on the enkephalin-induced anti-nociception in rats. Jpn. J. Pharmacol. 1998, 78, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Ito, K.; Maeda, M.; Akahori, K.; Takahashi, S.; Jin, X.L.; Matsuda, M.; Suzuki, T.; Oka, T.; Kobayashi, H. Activation of supraspinal NMDA receptors by both D-serine alone or in combination with morphine leads to the potentiation of antinociception in tail-flick test of rats. Eur. J. Pharmacol. 2007, 565, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Carstens, E.; Wilson, C. Rat tail flick reflex: Magnitude measurement of stimulus-response function, suppression by morphine and habituation. J. Neurophysiol. 1993, 70, 630–639. [Google Scholar] [CrossRef] [PubMed]
- d′Amore, A.; Chiarotti, F.; Renzi, P. High-intensity nociceptive stimuli minimize behavioral effects induced by restraining stress during the tail-flick test. J. Pharmacol. Toxicol. Methods 1992, 27, 197–201. [Google Scholar] [CrossRef]
- Elhabazi, K.; Ayachi, S.; Ilien, B.; Simonin, F. Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities. JoVE J. Vis. Exp. 2014, 89, e51264. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, K.; Akahori, K.; Yano, H.; Iwao, K.; Oka, T. Effects of peptidase inhibitors on anti-nociceptive action of dynorphin-(1–8) in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 361, 273–278. [Google Scholar] [CrossRef]
- Sontakke, S.D.; Jaiswal, S.R. Experimental evaluation of analgesic and anti-inflammatory activity of simvastatin and atorvastatin. Indian J. Pharmacol. 2012, 44, 475–479. [Google Scholar] [CrossRef]
- Muhammad, N.; Rehman, N.; Khan, H.; Saeed, M.; Gilani, A.H. Prokinetic and laxative effects of the crude methanolic extract of Viola betonicifolia whole plant in rodents. BMC Complement. Altern. Med. 2013, 13, 70. [Google Scholar] [CrossRef] [Green Version]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar]
- Vogel, H.G.; Vogel, W.H. Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- WOOLFE, G.; MacDonald, A. The evaluation of the analgesic action of pethidine hydrochloride (Demerol). J. Pharmacol. Exp. Ther. 1944, 80, 300–307. [Google Scholar]
- Ankier, S.I. New hot plate tests to quantify antinociceptive and narcotic antagonist activities. Eur. J. Pharmacol. 1974, 27, 1–4. [Google Scholar] [CrossRef]
- Bardo, M.T.; Hughes, R.A. Exposure to a nonfunctional hot plate as a factor in the assessment of morphine-induced analgesia and analgesic tolerance in rats. Pharmacol. Biochem. Behav. 1979, 10, 481–485. [Google Scholar] [CrossRef]
- Gamble, G.D.; Milne, R.J. Repeated exposure to sham testing procedures reduces reflex withdrawal and hot-plate latencies: Attenuation of tonic descending inhibition? Neurosci. Lett. 1989, 96, 312–317. [Google Scholar] [CrossRef]
- Van Ree, J.M.; Leys, A. Behavioral effects of morphine and phencyclidine in rats: The influence of repeated testing before and after single treatment. Eur. J. Pharmacol. 1985, 113, 353–362. [Google Scholar] [CrossRef]
- Jayanthi, M.; Jyoti, M. Experimental animal studies on analgesic and anti-nociceptive activity of Allium sativum (garlic) powder. Indian J. Res. Rep. Med. Sci. 2012, 2, 1–7. [Google Scholar]
- Carlsson, K.H.; Jurna, I. Depression by flupirtine, a novel analgesic agent, of motor and sensory responses of the nociceptive system in the rat spinal cord. Eur. J. Pharmacol. 1987, 143, 89–99. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Perkins, M.N.; Campbell, E.; Dray, A. Antinociceptive activity of the bradykinin B1 and B2 receptor antagonists, des-Arg9, [Leu8]-BK and HOE 140, in two models of persistent hyperalgesia in the rat. Pain 1993, 53, 191–197. [Google Scholar] [CrossRef]
- Cheah, M.; Fawcett, J.W.; Andrews, M.R. Assessment of thermal pain sensation in rats and mice using the Hargreaves test. Bio-Protocol 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banik, R.K.; Kabadi, R.A. A modified Hargreaves’ method for assessing threshold temperatures for heat nociception. J. Neurosci. Methods 2013, 219, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deuis, J.R.; Vetter, I. The thermal probe test: A novel behavioral assay to quantify thermal paw withdrawal thresholds in mice. Temperature 2016, 3, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portenoy, R.K.; Kanner, R. Pain Management: Theory and Practice; F.A. Davis: Philadelphia, PA, USA, 1996. [Google Scholar]
- Woolf, C.J. Pain. Neurobiol. Dis. 2000, 7, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Lavich, T.R.; Cordeiro, R.S.; Silva, P.M.; Martins, M.A. A novel hot-plate test sensitive to hyperalgesic stimuli and non-opioid analgesics. Braz. J. Med. Biol. Res. 2005, 38, 445–451. [Google Scholar] [CrossRef]
- Allchorne, A.J.; Broom, D.C.; Woolf, C.J. Detection of cold pain, cold allodynia and cold hyperalgesia in freely behaving rats. Mol. Pain 2005, 1, 36. [Google Scholar] [CrossRef] [Green Version]
- Attal, N.; Jazat, F.; Kayser, V.; Guilbaud, G. Further evidence for ‘pain-related’behaviours in a model of unilateral peripheral mononeuropathy. Pain 1990, 41, 235–251. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Boyce-Rustay, J.M.; Honore, P.; Jarvis, M.F. Animal models of acute and chronic inflammatory and nociceptive pain. Methods Mol. Biol. 2010, 617, 41–55. [Google Scholar] [CrossRef]
- Pizziketti, R.; Pressman, N.; Geller, E.; Cowan, A.; Adler, M. Rat cold water tail-flick: A novel analgesic test that distinguishes opioid agonists from mixed agonist-antagonists. Eur. J. Pharmacol. 1985, 119, 23–29. [Google Scholar] [CrossRef]
- Wang, J.J.; Ho, S.T.; Hu, O.Y.; Chu, K.M. An innovative cold tail-flick test: The cold ethanol tail-flick test. Anesth. Analg. 1995, 80, 102–107. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar]
- Anseloni, V.C.; Ennis, M.; Lidow, M.S. Optimization of the mechanical nociceptive threshold testing with the Randall–Selitto assay. J. Neurosci. Methods 2003, 131, 93–97. [Google Scholar] [CrossRef]
- Minett, M.S.; Quick, K.; Wood, J.N. Behavioral Measures of Pain Thresholds. Curr. Protoc. Mouse Biol. 2011, 1, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.P. Beta-endorphin immunoreactivity in rat plasma: Variations in response to different physical stimuli. Life Sci. 1981, 29, 1669–1674. [Google Scholar] [CrossRef]
- Romer, D. Pharmacological evaluation of mild analgesics. Br. J. Clin. Pharmacol. 1980, 10, 247S–251S. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.; Franceschini, J. Experimental observations on Haffner′s method for testing analgesic drugs. Br. J. Pharmacol. Chemother. 1954, 9, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Guria, T.; Singha, T.; Maity, T.K. Evaluation of Analgesic and Anti-Inflammatory Activity of Chloroform and Methanol Extracts of Centella asiatica Linn. ISRN Pharmacol. 2013, 2013, 789613. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Inukai, T.; Nakama, M. A modification of Haffner′s method for testing analgesics. Jpn. J. Pharmacol. 1966, 16, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Green, A.F.; Young, P.A. A comparison of heat and pressure analgesiometric methods in rats. Br. J. Pharmacol. Chemother. 1951, 6, 572–585. [Google Scholar] [CrossRef] [Green Version]
- Tyers, M. A classification of opiate receptors that mediate antinociception in animals. Br. J. Pharmacol. 1980, 69, 503–512. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.A.; Flataker, L. Reaction thresholds to pressure in edematous hindpaws of rats and responses to analgesic drugs. J. Pharmacol. Exp. Ther. 1965, 150, 165–171. [Google Scholar] [PubMed]
- Luis-Delgado, O.E.; Barrot, M.; Rodeau, J.L.; Schott, G.; Benbouzid, M.; Poisbeau, P.; Freund-Mercier, M.J.; Lasbennes, F. Calibrated forceps: A sensitive and reliable tool for pain and analgesia studies. J. Pain 2006, 7, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Barrot, M. Tests and models of nociception and pain in rodents. Neuroscience 2012, 211, 39–50. [Google Scholar] [CrossRef]
- Hans, G.H.; Vandervliet, E.; Deseure, K.; Parizel, P.M. Cerebral activation during von Frey filament stimulation in subjects with endothelin-1-induced mechanical hyperalgesia: A functional MRI study. BioMed Res. Int. 2013, 2013, 610727. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef]
- De Vry, D.J.; Barker, P.H.; Vardanyan, M.; Milosavljevic, S.L.; Dygert, T.N.; Jurva, J.W.; Wyler Van Ballmoos, M.C.; Gandhi, S.D.; Hossein Almassi, G.; Pagel, P.S. Pneumonia and Inflammatory Arthritis Caused by Unusual Occupational Exposure or a Life-Threatening Infection Resulting From a More Commonly Encountered Mechanism? J. Cardiothorac. Vasc. Anesth. 2015, 29, 1096–1099. [Google Scholar] [CrossRef]
- Weller, C.P.; Sulman, F.G. Drug action on tail shock-induced vocalization in mice and its relevance to analgesia. Eur. J. Pharmacol. 1970, 9, 227–234. [Google Scholar] [CrossRef]
- Eschalier, A.; Marty, H.; Trolese, J.F.; Moncharmont, L.; Fialip, J. An automated method to analyze vocalization of unrestrained rats submitted to noxious electrical stimuli. J. Pharmacol. Methods 1988, 19, 175–184. [Google Scholar] [CrossRef]
- Carroll, M.N.; Lim, R.K. Observations on the neuropharmacology of morphine and morphinelike analgesia. Arch. Int. Pharmacodyn. Ther. 1960, 125, 383–403. [Google Scholar]
- Weiss, B.; Laties, V.G. Fractional escape and avoidance on a titration schedule. Science 1958, 128, 1575–1576. [Google Scholar] [CrossRef]
- Graham, J.H.; Buccafusco, J.J. Inhibitory avoidance behavior and memory assessment. Methods Behav. Anal. Neurosci. 2001, 3, 141–151. [Google Scholar]
- Wahlsten, D. Standardizing tests of mouse behavior: Reasons, recommendations, and reality. Physiol. Behav. 2001, 73, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Helsley, G.C.; Richman, J.A.; Lunsford, C.D.; Jenkins, H.; Mays, R.P.; Funderburk, W.H.; Johnson, D.N. Analgetics. Esters of 3-pyrrolidinemethanols. J. Med. Chem. 1968, 11, 472–475. [Google Scholar] [CrossRef]
- Paalzow, G.; Paalzow, L. Morphine-induced inhibition of different pain responses in relation to the regional turnover of rat brain noradrenaline and dopamine. Psychopharmacologia 1975, 45, 9–20. [Google Scholar] [CrossRef]
- Vohel, H. Introduction Strategies in Drug Discovery and Evaluation. In Drug Discovery and Evaluation; Vohel, H.G., Ed.; Springer: New York, NY, USA, 2008; pp. 1–45. [Google Scholar]
- Wynn, R.L.; El′Baghdady, Y.M.; Ford, R.D.; Thut, P.D.; Rudo, F.G. A rabbit tooth-pulp assay to determine ED50 values and duration of action of analgesics. J. Pharmacol. Methods 1984, 11, 109–117. [Google Scholar] [CrossRef]
- Matthews, B.; Searle, B.N. Electrical stimulation of teeth. Pain 1976, 2, 245–251. [Google Scholar] [CrossRef]
- Dzoyem, J.; McGaw, L.; Kuete, V.; Bakowsky, U. Anti-inflammatory and anti-nociceptive activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–270. [Google Scholar]
- Tjolsen, A.; Berge, O.G.; Hunskaar, S.; Rosland, J.H.; Hole, K. The formalin test: An evaluation of the method. Pain 1992, 51, 5–17. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Jourdan, D.; Ardid, D.; Bardin, L.; Bardin, M.; Neuzeret, D.; Lanphouthacoul, L.; Eschalier, A. A new automated method of pain scoring in the formalin test in rats. Pain 1997, 71, 265–270. [Google Scholar] [CrossRef]
- Porro, C.A.; Cavazzuti, M. Spatial and temporal aspects of spinal cord and brainstem activation in the formalin pain model. Prog. Neurobiol. 1993, 41, 565–607. [Google Scholar] [CrossRef]
- Puig, S.; Sorkin, L.S. Formalin-evoked activity in identified primary afferent fibers: Systemic lidocaine suppresses phase-2 activity. Pain 1996, 64, 345–355. [Google Scholar] [CrossRef]
- Coderre, T.J.; Fundytus, M.E.; McKenna, J.E.; Dalal, S.; Melzack, R. The formalin test: A validation of the weighted-scores method of behavioural pain rating. Pain 1993, 54, 43–50. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Rai, G. Experimental evaluation of analgesic and anti-inflammatory potential of Oyster mushroom Pleurotus florida. Indian J. Pharmacol. 2013, 45, 66–70. [Google Scholar] [CrossRef]
- da Silva, G.F.; Rocha, L.W.; Quintão, N.L.M. Nutraceuticals, dietary supplements, and functional foods as alternatives for the relief of neuropathic pain. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 87–93. [Google Scholar]
- Gawade, S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, N. In-vivo models for management of pain. Pharmacol. Pharm. 2014, 5, 92. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.; Ghosh, G.; Kumar, P.S.; Panda, P.K. An experimental study of analgesic activity of selective COX-2 inhibitor with conventional NSAIDs. Asian J. Pharm Clin.Res. 2011, 4, 78–81. [Google Scholar]
- Franklin, K.B.; Abbott, F.V. Techniques for assessing the effects of drugs on nociceptive responses. In Psychopharmacology (Berl.); Springer: Berlin/Heidelberg, Germany, 1989; pp. 145–216. [Google Scholar]
- Siegmund, E.; Cadmus, R.; Lu, G. A method for evaluating both non-narcotic and narcotic analgesics. Proc. Soc. Exp. Biol. Med. 1957, 95, 729–731. [Google Scholar] [CrossRef]
- Matera, C.; Flammini, L.; Quadri, M.; Vivo, V.; Ballabeni, V.; Holzgrabe, U.; Mohr, K.; De Amici, M.; Barocelli, E.; Bertoni, S. Bis (ammonio) alkane-type agonists of muscarinic acetylcholine receptors: Synthesis, in vitro functional characterization, and in vivo evaluation of their analgesic activity. Eur. J. Med. Chem. 2014, 75, 222–232. [Google Scholar] [CrossRef]
- Iadarola, M.J.; Brady, L.S.; Draisci, G.; Dubner, R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: Stimulus specificity, behavioral parameters and opioid receptor binding. Pain 1988, 35, 313–326. [Google Scholar] [CrossRef]
- Millan, M.; Członkowski, A.; Morris, B.; Stein, C.; Arendt, R.; Huber, A.; Höllt, V.; Herz, A. Inflammation of the hind limb as a model of unilateral, localized pain: Influence on multiple opioid systems in the spinal cord of the rat. Pain 1988, 35, 299–312. [Google Scholar] [CrossRef]
- Zhang, R.X.; Lao, L.; Qiao, J.T.; Ruda, M.A. Strain differences in pain sensitivity and expression of preprodynorphin mRNA in rats following peripheral inflammation. Neurosci. Lett. 2003, 353, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.; Zhang, R.X.; Zhang, G.; Wang, X.; Berman, B.M.; Ren, K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004, 1020, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Rosenthale, M.E. A comparative study of the Lewis and Sprague Dawley rat in adjuvant arthritis. Arch. Int. Pharmacodyn. Ther. 1970, 188, 14–22. [Google Scholar]
- Gould III, H.J. Complete Freund′s adjuvant-induced hyperalgesia: A human perception. Pain 2000, 85, 301–303. [Google Scholar] [CrossRef]
- Clausen, B.E.; Laman, J.D. Inflammation: Methods and Protocols; Springer: New York, NY, USA, 2017. [Google Scholar]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Vinegar, R.; Schreiber, W.; Hugo, R. Biphasic development of carrageenin edema in rats. J. Pharmacol. Exp. Ther. 1969, 166, 96–103. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Inflammatory Models of Pain and Hyperalgesia. ILAR J. 1999, 40, 111–118. [Google Scholar] [CrossRef]
- Fecho, K.; Nackley, A.G.; Wu, Y.; Maixner, W. Basal and carrageenan-induced pain behavior in Sprague–Dawley, Lewis and Fischer rats. Physiol. Behav. 2005, 85, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Eastwood, B.J.; Need, A.B.; Shannon, H.E. Analgesic effects of serotonergic, noradrenergic or dual reuptake inhibitors in the carrageenan test in rats: Evidence for synergism between serotonergic and noradrenergic reuptake inhibition. Neuropharmacology 2006, 51, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; Baiuomy, A.R.; Arbid, M.S. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacol. Res. 2004, 49, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippe, I.T.; Stabentheiner, A.; Holzer, P. Participation of nitric oxide in the mustard oil-induced neurogenic inflammation of the rat paw skin. Eur. J. Pharmacol. 1993, 232, 113–120. [Google Scholar] [CrossRef]
- Urban, M.O.; Jiang, M.C.; Gebhart, G.F. Participation of central descending nociceptive facilitatory systems in secondary hyperalgesia produced by mustard oil. Brain Res. 1996, 737, 83–91. [Google Scholar] [CrossRef]
- Meller, S.; Gebhart, G. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur. J. Pain 1997, 1, 43–52. [Google Scholar] [CrossRef]
- LaMotte, R.H.; Shain, C.N.; Simone, D.A.; Tsai, E.F. Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. J. Neurophysiol. 1991, 66, 190–211. [Google Scholar] [CrossRef]
- Sumikura, H.; Andersen, O.K.; Drewes, A.M.; Arendt-Nielsen, L. Spatial and temporal profiles of flare and hyperalgesia after intradermal capsaicin. Pain 2003, 105, 285–291. [Google Scholar] [CrossRef]
- Serra, J.; Campero, M.; Ochoa, J. Flare and hyperalgesia after intradermal capsaicin injection in human skin. J. Neurophysiol. 1998, 80, 2801–2810. [Google Scholar] [CrossRef] [Green Version]
- Gilchrist, H.D.; Allard, B.L.; Simone, D.A. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain 1996, 67, 179–188. [Google Scholar] [CrossRef]
- Lariviere, W.R.; Melzack, R. The bee venom test: A new tonic-pain test. Pain 1996, 66, 271–277. [Google Scholar] [CrossRef]
- Chen, J.; Luo, C.; Li, H.-L.; Chen, H.-S. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: A comparative study with the formalin test. Pain 1999, 83, 67–76. [Google Scholar] [CrossRef]
- Combe, R.; Bramwell, S.; Field, M.J. The monosodium iodoacetate model of osteoarthritis: A model of chronic nociceptive pain in rats? Neurosci. Lett. 2004, 370, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Gegout-Pottie, P.; Philippe, L.; Simonin, M.A.; Guingamp, C.; Gillet, P.; Netter, P.; Terlain, B. Biotelemetry: An original approach to experimental models of inflammation. Inflamm. Res. 1999, 48, 417–424. [Google Scholar] [CrossRef]
- Otsuki, T.; Nakahama, H.; Niizuma, H.; Suzuki, J. Evaluation of the analgesic effects of capsaicin using a new rat model for tonic pain. Brain Res. 1986, 365, 235–240. [Google Scholar] [CrossRef]
- Sluka, K.A.; Milton, M.A.; Willis, W.D.; Westlund, K.N. Differential roles of neurokinin 1 and neurokinin 2 receptors in the development and maintenance of heat hyperalgesia induced by acute inflammation. Br. J. Pharmacol. 1997, 120, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Yam, M.F.; Por, L.Y.; Peh, K.K.; Ahmad, M.; Asmawi, M.Z.; Ang, L.F.; Yin, D.B.; Ong, S.Y.; Abdulkarim, M.F.; Abdullah, G.Z.; et al. Development of a stepping force analgesic meter for a rat arthritic model. Sensors 2011, 11, 5058–5070. [Google Scholar] [CrossRef]
- Tonussi, C.R.; Ferreira, S.H. Rat knee-joint carrageenin incapacitation test: An objective screen for central and peripheral analgesics. Pain 1992, 48, 421–427. [Google Scholar] [CrossRef]
- Swder, M.; Zakrocka, I.; Munir, E.; Swiader, K. Influence of Cimetidine in Combination with Antiepileptic Drugs on Locomotor Activity in Mice. Indian J. Pharm. Sci. 2019, 81, 776–781. [Google Scholar] [CrossRef]
- Sluka, K.A.; Westlund, K.N. Behavioral and immunohistochemical changes in an experimental arthritis model in rats. Pain 1993, 55, 367–377. [Google Scholar] [CrossRef]
- Gauldie, S.D.; McQueen, D.S.; Clarke, C.J.; Chessell, I.P. A robust model of adjuvant-induced chronic unilateral arthritis in two mouse strains. J. Neurosci. Methods 2004, 139, 281–291. [Google Scholar] [CrossRef]
- Skyba, D.A.; Radhakrishnan, R.; Sluka, K.A. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J. Pain 2005, 6, 41–47. [Google Scholar] [CrossRef]
- Yu, Y.C.; Koo, S.T.; Kim, C.H.; Lyu, Y.; Grady, J.J.; Chung, J.M. Two variables that can be used as pain indices in experimental animal models of arthritis. J. Neurosci. Methods 2002, 115, 107–113. [Google Scholar] [CrossRef]
- Han, J.S.; Bird, G.C.; Li, W.; Jones, J.; Neugebauer, V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J. Neurosci. Methods 2005, 141, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Aji, W. Preliminary study plate capacitor as a Plethysmometer sensor. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Mataram, Indonesia, 29–30 November 2017; p. 012036. [Google Scholar]
- Green, D.; Haines, J. A rat paw plethysmometer for single-handed operation. Med. Biol. Eng. 1966, 4, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Samud, A.M.; Asmawi, M.Z. Comparison between plethysmometer and micrometer methods to measure acute paw oedema for screening anti-inflammatory activity in mice. Inflammopharmacology 2004, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; Lacroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nature Methods 2010, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Akintola, T.; Raver, C.; Studlack, P.; Uddin, O.; Masri, R.; Keller, A. The grimace scale reliably assesses chronic pain in a rodent model of trigeminal neuropathic pain. Neurobiol. Pain 2017, 2, 13–17. [Google Scholar] [CrossRef]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [Green Version]

| Types of Stimuli | Test Methods | Benefits and Drawbacks (* Most Sensitive Analgesics) | Proposed Mechanismsfor Analgesia Profile Study | References |
|---|---|---|---|---|
| Phasic Pain (Nocifensive Tests) | ||||
| Thermal | 1. Tail-Flick
| Pros: Tail-Flick
Tail-Flick
| -Opioidergic -Adrenergic -Serotonergic -ASICs channels -TRP channels -Purinergic -Histaminergic -Cannabinoidergic | [10,11,12,13,14,15,16,17,18,19] |
| 2. Hot Plate | Pros:
| -Serotonergic -Adrenergic - NaV, CaV, KV and ClV channels -Nicotinergic -Opioidergic -COX -Cannabinoidergic | [10,17,18,20,21,22,23,24] | |
| 3. Paw Withdrawal/Hargreaves Test/Plantar Test | Pros:
| -Neurotrophin -Purinergic -Substance P -Histaminergic -Cannabinoidergic | [16,25,26,27,28,29,30,31,32,33] | |
| Mechanical | 1. Randal-Selitto | Pros:
| -Serotonergic -Cannabinoidergic -COX -ASICs channels -TRP channels -Substance P -Histaminergic -GABAergic | [16,24,34,35,36,37,38] |
| 2. Pricking Pain | Pros:
| -Serotonergic -Cannabinoidergic -COX | [24,38] | |
| 3. Haffner’s/Tail-Pinch | Pros:
| -Cannabinoidergic -COX -Opioidergic | [17,24] | |
| 4. Homemade Calibrated forceps | Pros:
| -Cannabinoidergic -COX -GABAergic | [12,24,39,40] | |
| 5. Von Frey Filament | Pros:
| -Neurotrophin -COX -Cannabinoidergic -NAV channel -TRP channels -ASICs channels -Purinergic -Substance P -CGRP -Histaminergic -GABAergic | [11,12,24,25,26,27,29,30,31,32,36,39,40,41,42,43,44,45,46,47,48,49,50,51,52] | |
| Electrical | 1. Shock-Induced Vocalization | Pros:
| -Serotonergic -COX -TTX-R Na channels | [47,52] |
| 2. Tooth-Pulp Stimulation | -Serotonergic -TTX-R Na channels | [47,52] | ||
| 3. Tail Shock/Nelson Test | -Serotonergic -Opiodergic -Purinergic -NMDA receptor -TTX-R Na channels | [47,52,53] | ||
| Tonic and Visceral Pain (Inflammatory Tests) | ||||
| Chemical | 1. Writhing Test | (* Peripherally acting analgesics) | -Serotonergic -Opioidergic -Peritoneal mast cells -ASICs channels -COX -GABAergic -Cannabinoidergic | [10,15,17,40,54,55,56] |
| 2. Formalin Test | (* Centrally acting analgesics) | Early phase -Serotonergic -Opioidergic -Substance P -Cannabinoidergic Late phase -Serotonergic -NO/cGMP/KATP pathways -Opioidergic -Histaminergic -COX -GABAergic -Cannabinoidergic | [15,17,40,56,57,58,59,60,61] | |
| Inflammatory Agents | Quantity Applied | Hyperalgesia | Allodynia | Onset of Action (within) | Duration of Action (≤) |
|---|---|---|---|---|---|
| λ Carrageenan | 100 µL of 1% (w/v) | + (t) | + (m) | 30 min | 3 days |
| Formalin | 50 µL of 5% (v/v) | + | + | 5 min | 60 min |
| Complete Freund’s Adjuvant | 1:1 dilution in phosphate buffered saline | + | + | 5 h | 2 weeks |
| Mustard Oil | 0.0625 - ≥ 5 mg | + | + | 5 min | 60 min |
| Zymosan | D | + (t/m) | + | 30 min | 24 h |
| Capsaicin | 10 µg/10µL in 10% Etol and 2-hydroxypropyl BETA cyclodextrin | + (t/m) | + | 1 min | 21 h |
| Venom | D | + (t) | + (m) | 1 min | 96 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yam, M.F.; Loh, Y.C.; Oo, C.W.; Basir, R. Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-Like” Behavioral Study with Proposed Analgesic Pathways. Int. J. Mol. Sci. 2020, 21, 4355. https://doi.org/10.3390/ijms21124355
Yam MF, Loh YC, Oo CW, Basir R. Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-Like” Behavioral Study with Proposed Analgesic Pathways. International Journal of Molecular Sciences. 2020; 21(12):4355. https://doi.org/10.3390/ijms21124355
Chicago/Turabian StyleYam, Mun Fei, Yean Chun Loh, Chuan Wei Oo, and Rusliza Basir. 2020. "Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-Like” Behavioral Study with Proposed Analgesic Pathways" International Journal of Molecular Sciences 21, no. 12: 4355. https://doi.org/10.3390/ijms21124355
APA StyleYam, M. F., Loh, Y. C., Oo, C. W., & Basir, R. (2020). Overview of Neurological Mechanism of Pain Profile Used for Animal “Pain-Like” Behavioral Study with Proposed Analgesic Pathways. International Journal of Molecular Sciences, 21(12), 4355. https://doi.org/10.3390/ijms21124355