Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back
Abstract
:1. Introduction
2. The Phyto-Cannabinoid Activity Is Mediated by the Endocannabinoid System and Other Physiological Signaling Systems
3. The Roles of the Endocannabinoid System in Immunity
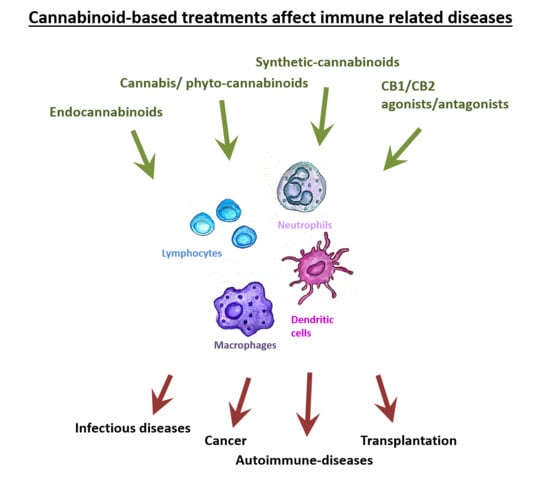
4. The Effects of Cannabinoid-Based Treatments in Different Immune Related Diseases
4.1. Infectious Diseases
4.2. Cancer
4.3. Autoimmune Diseases
4.4. Transplantation
5. How to Choose the Best Treatment?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, H.E.; Li, X.; Zhao, Y.X.; Ferguson, D.K.; Hueber, F.; Bera, S.; Wang, Y.F.; Zhao, L.C.; Liu, C.J.; Li, C.S. A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J. Ethnopharmacol. 2006, 108, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Bifulco, M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Bifulco, M. Modern History of Medical Cannabis: From Widespread Use to Prohibitionism and Back. Trends Pharm. Sci. 2017, 38, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanus, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzet, M.; Breton, C.; Bisogno, T.; Di Marzo, V. Comparative biology of the endocannabinoid system possible role in the immune response. Eur. J. Biochem. 2000, 267, 4917–4927. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [Green Version]
- Martínez, V.; Iriondo De-Hond, A.; Borrelli, F.; Capasso, R.; Del Castillo, M.D.; Abalo, R. Cannabidiol and Other Non-Psychoactive Cannabinoids for Prevention and Treatment of Gastrointestinal Disorders: Useful Nutraceuticals? Int. J. Mol. Sci. 2020, 21, 3067. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duc, N.M.; Kim, H.R.; Chung, K.Y. Structural mechanism of G protein activation by G protein-coupled receptor. Eur. J. Pharmacol. 2015, 763, 214–222. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A., 3rd; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [PubMed]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB(1). Nature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Parolaro, D. Presence and functional regulation of cannabinoid receptors in immune cells. Life Sci. 1999, 65, 637–644. [Google Scholar] [CrossRef]
- Demuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar]
- Olah, A.; Szekanecz, Z.; Biro, T. Targeting Cannabinoid Signaling in the Immune System: “High”-ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef] [Green Version]
- Dlugos, A.; Childs, E.; Stuhr, K.L.; Hillard, C.J.; de Wit, H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 2012, 37, 2416–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karhson, D.S.; Krasinska, K.M.; Dallaire, J.A.; Libove, R.A.; Phillips, J.M.; Chien, A.S.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 2018, 43, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol. Autism. 2019, 10, 2. [Google Scholar] [CrossRef]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging. 2013, 34, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018, 17, 623–639. [Google Scholar] [CrossRef]
- McPartland, J.M.; Glass, M.; Pertwee, R.G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. Br. J. Pharmacol. 2007, 152, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef] [Green Version]
- Kose, S.; Aerts-Kaya, F.; Kopru, C.Z.; Nemutlu, E.; Kuskonmaz, B.; Karaosmanoglu, B.; Taskiran, E.Z.; Altun, B.; Uckan Cetinkaya, D.; Korkusuz, P. Human bone marrow mesenchymal stem cells secrete endocannabinoids that stimulate in vitro hematopoietic stem cell migration effectively comparable to beta-adrenergic stimulation. Exp. Hematol. 2018, 57, 30–41.e1. [Google Scholar] [CrossRef]
- Pereira, J.P.; An, J.; Xu, Y.; Huang, Y.; Cyster, J.G. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat. Immunol. 2009, 10, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Pelus, L.M. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia 2010, 24, 1993–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khuja, I.; Yekhtin, Z.; Or, R.; Almogi-Hazan, O. Cannabinoids Reduce Inflammation but Inhibit Lymphocyte Recovery in Murine Models of Bone Marrow Transplantation. Int. J. Mol. Sci. 2019, 20, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabady, R.L.; Louissaint, C.; Lubben, A.; Xie, B.; Reeksting, S.; Tuohy, C.; Demma, Z.; Foley, S.E.; Faherty, C.S.; Llanos-Chea, A.; et al. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J. Clin. Invest. 2018, 128, 4044–4056. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Taylor, L.; Feuerborn, A.; Valaris, S.; Hussain, M.T.; Rainger, G.E.; Greaves, D.R.; Iqbal, A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. Faseb. J. 2019, 33, 6154–6167. [Google Scholar] [CrossRef] [Green Version]
- Tahamtan, A.; Samieipoor, Y.; Nayeri, F.S.; Rahbarimanesh, A.A.; Izadi, A.; Rashidi-Nezhad, A.; Tavakoli-Yaraki, M.; Farahmand, M.; Bont, L.; Shokri, F.; et al. Effects of cannabinoid receptor type 2 in respiratory syncytial virus infection in human subjects and mice. Virulence 2018, 9, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.F.; Newton, C.; Widen, R.; Friedman, H.; Klein, T.W. Differential expression of cannabinoid CB(2) receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharm. 2001, 423, 235–241. [Google Scholar] [CrossRef]
- Sugamura, K.; Sugiyama, S.; Nozaki, T.; Matsuzawa, Y.; Izumiya, Y.; Miyata, K.; Nakayama, M.; Kaikita, K.; Obata, T.; Takeya, M.; et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009, 119, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Chiurchiu, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Miranda, K.; Mehrpouya-Bahrami, P.; Nagarkatti, P.S.; Nagarkatti, M. Cannabinoid Receptor 1 Blockade Attenuates Obesity and Adipose Tissue Type 1 Inflammation Through miR-30e-5p Regulation of Delta-Like-4 in Macrophages and Consequently Downregulation of Th1 Cells. Front. Immunol. 2019, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, W.; Shi, R.; Kang, X.; Zhang, X.; Chen, P.; Zhang, L.; Hou, A.; Wang, R.; Zhao, Y.; Zhao, K.; et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat. Commun. 2018, 9, 2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, S.; Kocieda, V.P.; Yen, J.H.; Tuma, R.F.; Ganea, D. Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression. Blood 2012, 120, 3741–3749. [Google Scholar] [CrossRef] [Green Version]
- Maestroni, G.J. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. Faseb. J. 2004, 18, 1914–1916. [Google Scholar] [CrossRef] [Green Version]
- Gardner, B.; Zu, L.X.; Sharma, S.; Liu, Q.; Makriyannis, A.; Tashkin, D.P.; Dubinett, S.M. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem. Biophys. Res. Commun. 2002, 290, 91–96. [Google Scholar] [CrossRef]
- Robinson, R.H.; Meissler, J.J.; Breslow-Deckman, J.M.; Gaughan, J.; Adler, M.W.; Eisenstein, T.K. Cannabinoids inhibit T-cells via cannabinoid receptor 2 in an in vitro assay for graft rejection, the mixed lymphocyte reaction. J. Neuroimmune Pharm. 2013, 8, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Borner, C.; Hollt, V.; Kraus, J. Activation of human T cells induces upregulation of cannabinoid receptor type 1 transcription. Neuroimmunomodulation 2007, 14, 281–286. [Google Scholar]
- Robinson, R.H.; Meissler, J.J.; Fan, X.; Yu, D.; Adler, M.W.; Eisenstein, T.K. A CB2-Selective Cannabinoid Suppresses T-Cell Activities and Increases Tregs and IL-10. J. Neuroimmune Pharm. 2015, 10, 318–332. [Google Scholar] [CrossRef]
- Borner, C.; Smida, M.; Hollt, V.; Schraven, B.; Kraus, J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J. Biol. Chem. 2009, 284, 35450–35460. [Google Scholar] [CrossRef] [Green Version]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Sumida, H.; Lu, E.; Chen, H.; Yang, Q.; Mackie, K.; Cyster, J.G. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenstein, T.K.; Meissler, J.J.; Wilson, Q.; Gaughan, J.P.; Adler, M.W. Anandamide and Delta9-tetrahydrocannabinol directly inhibit cells of the immune system via CB2 receptors. J. Neuroimmunol. 2007, 189, 17–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Production of endocannabinoids by activated T cells and B cells modulates inflammation associated with delayed-type hypersensitivity. Eur. J. Immunol. 2016, 46, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- Dotsey, E.; Ushach, I.; Pone, E.; Nakajima, R.; Jasinskas, A.; Argueta, D.A.; Dillon, A.; DiPatrizio, N.; Davies, H.; Zlotnik, A.; et al. Transient Cannabinoid Receptor 2 Blockade during Immunization Heightens Intensity and Breadth of Antigen-specific Antibody Responses in Young and Aged mice. Sci. Rep. 2017, 7, 42584. [Google Scholar] [CrossRef] [Green Version]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [CrossRef]
- Zgair, A.; Lee, J.B.; Wong, J.C.M.; Taha, D.A.; Aram, J.; Di Virgilio, D.; McArthur, J.W.; Cheng, Y.K.; Hennig, I.M.; Barrett, D.A.; et al. Oral administration of Cannabis with lipids leads to high levels of cannabinoids in the intestinal lymphatic system and prominent immunomodulation. Sci. Rep. 2017, 7, 14542. [Google Scholar] [CrossRef]
- Bradley, S.G.; Munson, A.E.; Dewey, W.L.; Harris, L.S. Enhanced susceptibility of mice to combinations of delta 9-tetrahydrocannabinol and live or killed gram-negative bacteria. Infect. Immun. 1977, 17, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Morahan, P.S.; Klykken, P.C.; Smith, S.H.; Harris, L.S.; Munson, A.E. Effects of cannabinoids on host resistance to Listeria monocytogenes and herpes simplex virus. Infect. Immun. 1979, 23, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Cabral, G.A.; Rogers, T.J.; Lichtman, A.H. Turning Over a New Leaf: Cannabinoid and Endocannabinoid Modulation of Immune Function. J. Neuroimmune Pharm. 2015, 10, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabral, G.A.; Dove Pettit, D.A. Drugs and immunity: Cannabinoids and their role in decreased resistance to infectious disease. J. Neuroimmunol. 1998, 83, 116–123. [Google Scholar] [CrossRef]
- Klein, T.W.; Friedman, H.; Specter, S. Marijuana, immunity and infection. J. Neuroimmunol. 1998, 83, 102–115. [Google Scholar] [CrossRef]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez-Cervantes, R.; Mendez-Diaz, M.; Prospero-Garcia, O.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Coppola, N.; Zampino, R.; Sagnelli, C.; Bellini, G.; Marrone, A.; Stanzione, M.; Capoluongo, N.; Boemio, A.; Minichini, C.; Adinolfi, L.E.; et al. Cannabinoid receptor 2-63 QQ variant is associated with persistently normal aminotransferase serum levels in chronic hepatitis C. PloS ONE 2014, 9, e99450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagnelli, C.; Uberti-Foppa, C.; Hasson, H.; Bellini, G.; Minichini, C.; Salpietro, S.; Messina, E.; Barbanotti, D.; Merli, M.; Punzo, F.; et al. In vivo evidence that the cannabinoid receptor 2-63 RR variant is associated with the acquisition and/or expansion of HIV infection. HIV Med. 2018, 19, 597–604. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, A.; Tavakoli-Yaraki, M.; Rygiel, T.P.; Mokhtari-Azad, T.; Salimi, V. Effects of cannabinoids and their receptors on viral infections. J. Med. Virol. 2016, 88, 1–12. [Google Scholar] [CrossRef]
- Blevins, R.D.; Dumic, M.P. The effect of delta-9-tetrahydrocannabinol on herpes simplex virus replication. J. Gen. Virol. 1980, 49, 427–431. [Google Scholar] [CrossRef]
- Medveczky, M.M.; Sherwood, T.A.; Klein, T.W.; Friedman, H.; Medveczky, P.G. Delta-9 tetrahydrocannabinol (THC) inhibits lytic replication of gamma oncogenic herpesviruses in vitro. BMC Med. 2004, 2, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinola, O.; Ogbeche, E.O.; Olumoh-Abdul, H.A.; Alli-Oluwafuyi, A.O.; Oyewole, A.L.; Amin, A.; AbdulMajeed, W.I.; Olajide, O.J.; Nafiu, A.B.; Njan, A.A.; et al. Oral Ingestion of Cannabis sativa: Risks, Benefits, and Effects on Malaria-Infected Hosts. Cannabis Cannabinoid Res. 2018, 3, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Batugedara, H.M.; Argueta, D.; Jang, J.C.; Lu, D.; Macchietto, M.; Kaur, J.; Ge, S.; Dillman, A.R.; DiPatrizio, N.V.; Nair, M.G. Host- and Helminth-Derived Endocannabinoids That Have Effects on Host Immunity Are Generated during Infection. Infect. Immun. 2018, 86, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiertscher, S.M.; Gangalum, P.R.; Ibrahim, G.; Tashkin, D.P.; Roth, M.D. A Prospective Study of Humoral and Cellular Immune Responses to Hepatitis B Vaccination in Habitual Marijuana Smokers. J. Neuroimmune Pharm. 2018, 13, 219–229. [Google Scholar] [CrossRef]
- Brown, D.; Watson, M.; Schloss, J. Pharmacological evidence of medicinal Cannabis in oncology: A systematic review. Support Care Cancer 2019, 27, 3195–3207. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sanchez, C.; Guzman, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091–4106. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Yang, L.; Wang, B.; Cui, L.; Li, C.; Zhuo, Y.; Zhang, L.; Zhang, S.; Zhang, Q.; Wang, X. The role of 2-arachidonoylglycerol in the regulation of the tumor-immune microenvironment in murine models of pancreatic cancer. Biomed Pharm. 2019, 115, 108952. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Moreno, E.; Seijo-Vila, M.; Tundidor, I.; Andradas, C.; Caffarel, M.M.; Caro-Villalobos, M.; Uriguen, L.; Diez-Alarcia, R.; Moreno-Bueno, G.; et al. Therapeutic targeting of HER2-CB2R heteromers in HER2-positive breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3863–3872. [Google Scholar] [CrossRef] [Green Version]
- Ladin, D.A.; Soliman, E.; Griffin, L.; Van Dross, R. Preclinical and Clinical Assessment of Cannabinoids as Anti-Cancer Agents. Front. Pharm. 2016, 7, 361. [Google Scholar] [CrossRef] [Green Version]
- Moreno, E.; Cavic, M.; Krivokuca, A.; Casado, V.; Canela, E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front. Pharm. 2019, 10, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraguas-Sanchez, A.I.; Martin-Sabroso, C.; Torres-Suarez, A.I. Insights into the effects of the endocannabinoid system in cancer: A review. Br. J. Pharm. 2018, 175, 2566–2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [Green Version]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. 2000, 165, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernandez-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzman, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef] [Green Version]
- Taha, T.; Meiri, D.; Talhamy, S.; Wollner, M.; Peer, A.; Bar-Sela, G. Cannabis Impacts Tumor Response Rate to Nivolumab in Patients with Advanced Malignancies. Oncologist 2019, 24, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.; Katz, I.; Porat-Katz, B.S.; Shoenfeld, Y. Medical Cannabis: Another piece in the mosaic of autoimmunity? Clin. Pharm. 2017, 101, 230–238. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharm. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- Gentili, M.; Ronchetti, S.; Ricci, E.; Di Paola, R.; Gugliandolo, E.; Cuzzocrea, S.; Bereshchenko, O.; Migliorati, G.; Riccardi, C. Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharm. Res. 2019, 141, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive Cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, H.; Liu, X.; Liu, L.R.; Su, D.F.; Dai, S.M. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology 2015, 220, 817–822. [Google Scholar] [CrossRef]
- Lowin, T.; Apitz, M.; Anders, S.; Straub, R.H. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res. 2015, 17, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, J.J.; Muley, M.M.; Philpott, H.T.; Reid, A.; Krustev, E. Early blockade of joint inflammation with a fatty acid amide hydrolase inhibitor decreases end-stage osteoarthritis pain and peripheral neuropathy in mice. Arthritis Res. 2017, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. 2008, 10, R43. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.K.P. A perspective review on fatty acid amide hydrolase (FAAH) inhibitors as potential therapeutic agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Kerbrat, A.; Ferré, J.C.; Fillatre, P.; Ronzière, T.; Vannier, S.; Carsin-Nicol, B.; Lavoué, S.; Vérin, M.; Gauvrit, J.Y.; Le Tulzo, Y.; et al. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. 2016, 375, 1717–1725. [Google Scholar] [CrossRef] [Green Version]
- Blake, D.R.; Robson, P.; Ho, M.; Jubb, R.W.; McCabe, C.S. Preliminary assessment of the efficacy, tolerability and safety of a Cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology 2006, 45, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Lyman, W.D.; Sonett, J.R.; Brosnan, C.F.; Elkin, R.; Bornstein, M.B. Delta 9-tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1989, 23, 73–81. [Google Scholar] [CrossRef]
- Pryce, G.; Riddall, D.R.; Selwood, D.L.; Giovannoni, G.; Baker, D. Neuroprotection in Experimental Autoimmune Encephalomyelitis and Progressive Multiple Sclerosis by Cannabis-Based Cannabinoids. J. Neuroimmune Pharm. 2015, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharm. 2011, 163, 1507–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, M.; Cudaback, E.; Abdullah, R.A.; Finnell, J.; Mischley, L.K.; Rozga, M.; Lichtman, A.H.; Stella, N. Cannabis use by individuals with multiple sclerosis: Effects on specific immune parameters. Inflammopharmacology 2014, 22, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Rovare, V.P.; Magalhaes, G.P.A.; Jardini, G.D.A.; Beraldo, M.L.; Gameiro, M.O.; Agarwal, A.; Luvizutto, G.J.; Paula-Ramos, L.; Camargo, S.E.A.; de Oliveira, L.D.; et al. Cannabinoids for spasticity due to multiple sclerosis or paraplegia: A systematic review and meta-analysis of randomized clinical trials. Complement Med. 2017, 34, 170–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akgun, K.; Essner, U.; Seydel, C.; Ziemssen, T. Daily Practice Managing Resistant Multiple Sclerosis Spasticity With Delta-9-Tetrahydrocannabinol: Cannabidiol Oromucosal Spray: A Systematic Review of Observational Studies. J Cent Nerv. Syst. Dis. 2019, 11, 1179573519831997. [Google Scholar] [CrossRef]
- Leinwand, K.L.; Gerich, M.E.; Hoffenberg, E.J.; Collins, C.B. Manipulation of the Endocannabinoid System in Colitis: A Comprehensive Review. Inflamm. Bowel Dis. 2017, 23, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Storr, M.A.; Keenan, C.M.; Emmerdinger, D.; Zhang, H.; Yüce, B.; Sibaev, A.; Massa, F.; Buckley, N.E.; Lutz, B.; Göke, B.; et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: Involvement of CB1 and CB2 receptors. J. Mol. Med. 2008, 86, 925–936. [Google Scholar] [CrossRef]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [Green Version]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef] [Green Version]
- Ambrose, T.; Simmons, A. Cannabis, Cannabinoids, and the Endocannabinoid System-Is there Therapeutic Potential for Inflammatory Bowel Disease? J. Crohns Colitis 2019, 13, 525–535. [Google Scholar] [CrossRef]
- Naftali, T.; Bar-Lev Schleider, L.; Dotan, I.; Lansky, E.P.; Sklerovsky Benjaminov, F.; Konikoff, F.M. Cannabis induces a clinical response in patients with Crohn’s disease: A prospective placebo-controlled study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1. [Google Scholar] [CrossRef] [PubMed]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Mbachi, C.; Attar, B.; Oyenubi, O.; Yuchen, W.; Efesomwan, A.; Paintsil, I.; Madhu, M.; Ajiboye, O.; Simons-Linares, C.R.; Trick, W.E.; et al. Association between Cannabis use and complications related to ulcerative colitis in hospitalized patients: A propensity matched retrospective cohort study. Medicine 2019, 98, e16551. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo-A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef]
- Hegde, V.L.; Hegde, S.; Cravatt, B.F.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.S. Attenuation of experimental autoimmune hepatitis by exogenous and endogenous cannabinoids: Involvement of regulatory T cells. Mol. Pharm. 2008, 74, 20–33. [Google Scholar] [CrossRef]
- Morsch, M.; Protti, D.A.; Cheng, D.; Braet, F.; Chung, R.S.; Reddel, S.W.; Phillips, W.D. Cannabinoid-induced increase of quantal size and enhanced neuromuscular transmission. Sci. Rep. 2018, 8, 4685. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, C.L.; Chen, M.; Manivannan, A.; Cabay, L.; Pertwee, R.G.; Coutts, A.; Forrester, J.V. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007, 82, 532–541. [Google Scholar] [CrossRef]
- Katz-Talmor, D.; Kivity, S.; Blank, M.; Katz, I.; Perry, O.; Volkov, A.; Barshack, I.; Amital, H.; Shoenfeld, Y. Cannabidiol Treatment in a Murine Model of Systemic Lupus Erythematosus Accelerates Proteinuria Development. Isr. Med. Assoc. J. 2018, 20, 741. [Google Scholar]
- Navarini, L.; Bisogno, T.; Mozetic, P.; Piscitelli, F.; Margiotta, D.P.E.; Basta, F.; Afeltra, A.; Maccarrone, M. Endocannabinoid system in systemic lupus erythematosus: First evidence for a deranged 2-arachidonoylglycerol metabolism. Int. J. Biochem. Cell Biol. 2018, 99, 161–168. [Google Scholar] [CrossRef]
- Rahaman, O.; Bhattacharya, R.; Liu, C.S.C.; Raychaudhuri, D.; Ghosh, A.R.; Bandopadhyay, P.; Pal, S.; Goswami, R.P.; Sircar, G.; Ghosh, P.; et al. Cutting Edge: Dysregulated Endocannabinoid-Rheostat for Plasmacytoid Dendritic Cell Activation in a Systemic Lupus Endophenotype. J. Immunol. 2019, 202, 1674–1679. [Google Scholar] [CrossRef] [Green Version]
- Imtiaz, S.; Rehm, J. The relationship between Cannabis use and diabetes: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Drug Alcohol Rev. 2018, 37, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.K.; Lundin, A.; Yaregal, A.; Ostenson, C.G.; Allebeck, P.; Agardh, E.E. Cannabis Use as Risk or Protection for Type 2 Diabetes: A Longitudinal Study of 18 000 Swedish Men and Women. J. Diabetes Res. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarkatti, M.; Rieder, S.A.; Hegde, V.L.; Kanada, S.; Nagarkatti, P. Do cannabinoids have a therapeutic role in transplantation? Trends Pharm. Sci. 2010, 31, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, H.S.; Winder, G.S. Marijuana Use and Organ Transplantation: A Review and Implications for Clinical Practice. Curr. Psychiatry Rep. 2017, 19, 91. [Google Scholar] [CrossRef]
- Pandey, R.; Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Targeting cannabinoid receptors as a novel approach in the treatment of graft-versus-host disease: Evidence from an experimental murine model. J. Pharm. Exp. 2011, 338, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Delta(9)-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshurun, M.; Shpilberg, O.; Herscovici, C.; Shargian, L.; Dreyer, J.; Peck, A.; Israeli, M.; Levy-Assaraf, M.; Gruenewald, T.; Mechoulam, R.; et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol. Blood Marrow Transpl. 2015, 21, 1770–1775. [Google Scholar] [CrossRef] [Green Version]
- Cunetti, L.; Manzo, L.; Peyraube, R.; Arnaiz, J.; Curi, L.; Orihuela, S. Chronic Pain Treatment With Cannabidiol in Kidney Transplant Patients in Uruguay. Transpl. Proc. 2018, 50, 461–464. [Google Scholar] [CrossRef]
- Greenan, G.; Ahmad, S.B.; Anders, M.G.; Leeser, A.; Bromberg, J.S.; Niederhaus, S.V. Recreational marijuana use is not associated with worse outcomes after renal transplantation. Clin. Transpl. 2016, 30, 1340–1346. [Google Scholar] [CrossRef]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol attenuates Chemically-induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharm. 2016, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. A cannabigerol-rich Cannabis sativa extract, devoid of [INCREMENT]9-tetrahydrocannabinol, elicits hyperphagia in rats. Behav. Pharm. 2017, 28, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential Cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharm. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Kamal, B.S.; Kamal, F.; Lantela, D.E. Cannabis and the Anxiety of Fragmentation-A Systems Approach for Finding an Anxiolytic Cannabis Chemotype. Front Neurosci. 2018, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.J.A.; Freeman, T.P.; Hindocha, C.; Schafer, G.; Gardner, C.; Curran, H.V. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl. Psychiatry 2018, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Chatkin, J.M.; Zani-Silva, L.; Ferreira, I.; Zamel, N. Cannabis-Associated Asthma and Allergies. Clin. Rev. Allergy Immunol. 2019, 56, 196–206. [Google Scholar] [CrossRef]
- Decuyper, II; Van Gasse, A.L.; Cop, N.; Sabato, V.; Faber, M.A.; Mertens, C.; Bridts, C.H.; Hagendorens, M.M.; De Clerck, L.; Rihs, H.P.; et al. Cannabis sativa allergy: Looking through the fog. Allergy 2017, 72, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Silvers, W.S.; Bernard, T. Spectrum and prevalence of reactions to marijuana in a Colorado allergy practice. Ann. Allergy Asthma Immunol. 2017, 119, 570–571. [Google Scholar] [CrossRef]
- Ebo, D.G.; Swerts, S.; Sabato, V.; Hagendorens, M.M.; Bridts, C.H.; Jorens, P.G.; De Clerck, L.S. New food allergies in a European non-Mediterranean region: Is Cannabis sativa to blame? Int. Arch. Allergy Immunol. 2013, 161, 220–228. [Google Scholar] [CrossRef]


| Indication | Treatment | Bot/syn | Phase | Status (Updated on) | ClinicalTrials.gov Identifiers/EudraCT Number |
|---|---|---|---|---|---|
| Inflammation (Observational study) | Cannabis | bot | n.a. | Recruiting (August 2018) | NCT03522103 |
| Cachexia; Advanced Cancer | THC/CBD | syn | Phase 3 | Not yet recruiting (May 2020) | NCT04001010 |
| Glioblastoma; Cancer | THC/CBD | n.a. | Phase 1-2 | Not yet recruiting (April 2020) | NCT03529448 |
| Chemotherapy induced Nausea and Vomiting; Pancreatic Cancer | Dronabinol | syn | Phase 3 | Recruiting (April 2020) | NCT03984214 2019-000616-28 |
| Chemotherapy induced Peripheral Neuropathy; Cancer | Cannabinoids | n.a. | Phase 2 | Recruiting (September 2019) | NCT03782402 |
| Cancer cachexia; Pancreatic cancer | Cannibinols | syn | n.a. | Ongoing (January 2018) | 2017-000530-54 |
| Solid Tumor; Cancer | Cannabis | bot | n.a. | Recruiting (March 2020) | NCT03617692 |
| Rheumatoid arthritis | THC/CBD | bot | n.a. | Temporarily Halted (October 2018) | 2017-004226-15 |
| Spasticity Due to Multiple Sclerosis | BX-1 | bot | Phase 3 | Recruiting (January 2020) | NCT03756974 |
| Inflammatory Bowel Diseases | Nabilone | syn | n.a. | Not yet recruiting (February 2020) | NCT03422861 |
| Crohn’s Disease | THC/CBD | n.a. | Phase 1-2 | Completed (March 2019) | NCT01826188 |
| Systemic Lupus Erythematosus | JBT-101 | syn | Phase 2 | Recruiting (March 2020) | NCT03093402 |
| Type 2 Diabetes | Cannabis | bot | n.a. | Recruiting (March 2020) | NCT04114903 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. https://doi.org/10.3390/ijms21124448
Almogi-Hazan O, Or R. Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back. International Journal of Molecular Sciences. 2020; 21(12):4448. https://doi.org/10.3390/ijms21124448
Chicago/Turabian StyleAlmogi-Hazan, Osnat, and Reuven Or. 2020. "Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back" International Journal of Molecular Sciences 21, no. 12: 4448. https://doi.org/10.3390/ijms21124448
APA StyleAlmogi-Hazan, O., & Or, R. (2020). Cannabis, the Endocannabinoid System and Immunity—the Journey from the Bedside to the Bench and Back. International Journal of Molecular Sciences, 21(12), 4448. https://doi.org/10.3390/ijms21124448