Maackiain Ameliorates 6-Hydroxydopamine and SNCA Pathologies by Modulating the PINK1/Parkin Pathway in Models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y Cell Line
Abstract
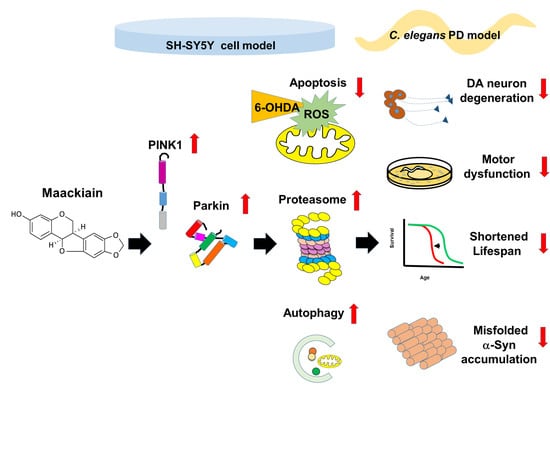
:1. Introduction
2. Results
2.1. Using the Food Clearance Test to Determine the Concentration Range of Maackiain Treatment in C. Elegans
2.2. Maackiain Diminished Dopaminergic Neuron Degeneration Caused by 6-OHDA Exposure in Worms
2.3. Recovery of Food-Sensing Behavior by Maackiain Treatment in 6-OHDA-Exposed Worms
2.4. Maackiain Treatment Augments Life-Span of 6-OHDA-Exposed Worms
2.5. α-Synuclein Protein Accumulation Was Diminished by Maackiain Treatment
2.6. Reduction in Dopaminergic Neuron Degeneration in 6-OHDA-Exposed Worm Model by Maackiain Treatment Linked to Reactive Oxygen Species Level and Expression of Pink1 and Pdr-1
2.7. Maackiain Lessened α-Synuclein Accumulation through Pdr-1 (Parkin) Expression to Enhance Somatic Proteasome Activity and Autophagy
2.8. The Ability of Maackiain to Improve PD Pathology Can Be Abolished by Downregulating the Expression of Pdr-1
2.9. Parkin siRNA Reversed Maackiain-Mediated Anti-Apoptosis in the 6-OHDA-Exposed SH-SY5Y Cell Line
2.10. Parkin siRNA Obstructed Maackiain-Mediated Enhancing of Ubiquitin-Proteasome System and Induction of Autophagy in an α-Synuclein-Overexpressing SH-SY5Y Cell Line
3. Discussion
4. Materials and Methods
4.1. Chemicals, C. elegans Strains, and Worm Synchronization
4.2. Food Clearance Test
4.3. 6-OHDA-Induced Dopaminergic Neuron Degeneration and Maackiain Treatment
4.4. Analysis of Dopaminergic Neuron Degeneration
4.5. Food-Sensing Behavior Test
4.6. Life-Span Test
4.7. Analysis of α-Synuclein Accumulation
4.8. Analysis of Protein Expression of C. elegans
4.9. Reactive Oxygen Species Assay
4.10. RNA Extraction and qPCR of C. elegans
4.11. Proteasome Activity Assays of C. elegans
4.12. Autophagy Activity Assay of C. elegans
4.13. RNA-mediated interference of C. elegans
4.14. Culture of the SH-SY5Y Cell Line and Treatment with 6-OHDA
4.15. Generation of an SH-SY5Y Cell Line Transiently Overexpressing α-Synuclein
4.16. Small RNA Interference of the SH-SY5Y Cell Line
4.17. Western Blot Analysis of the SH-SY5Y Cell Line
4.18. Nuclear Staining of Hoechst 33,258 and Measurement of Mitochondrial Membrane Potential in the SH-SY5Y Cell Line
4.19. Immunofluorescence Staining
4.20. Proteasome Activity Assay and Acidic Vesicular Organelle Staining in the SH-SY5Y Cell Line
4.21. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DMSO | Dimethyl sulfoxide |
| GFP | green fluorescent protein |
| MK | Maackiain |
| MMP | Mitochondrial membrane potential |
| MPTP | Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| 6-OHDA | 6-Hydroxydopamine |
| PD | Parkinson’s disease |
| PINK1 | Phosphatase and tension homologue (PTEN)-induced kinase 1 |
| siRNA | small interfering RNA |
| α-Syn | α-synuclein |
| UPS | ubiquitin-proteasome system |
| YFP | yellow fluorescent protein |
References
- Calabrese, V.; Santoro, A.; Monti, D.; Crupi, R.; Di Paola, R.; Latteri, S.; Cuzzocrea, S.; Zappia, M.; Giordano, J.; Calabrese, E.J.; et al. Aging and Parkinson’s Disease: Inflammaging, neuroinflammation and biological remodeling as key factors in pathogenesis. Free. Radic. Biol. Med. 2018, 115, 80–91. [Google Scholar] [CrossRef]
- Bouca-Machado, R.; Duarte, G.S.; Patriarca, M.; Castro Caldas, A.; Alarcao, J.; Fernandes, R.M.; Mestre, T.A.; Matias, R.; Ferreira, J.J. Measurement Instruments to Assess Functional Mobility in Parkinson’s Disease: A Systematic Review. Mov. Disord. Clin. Pract. 2020, 7, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrageiro, G.; Haylett, W.; Seedat, S.; Kuivaniemi, H.; Bardien, S. A review of genome-wide transcriptomics studies in Parkinson’s disease. Eur. J. Neurosci. 2018, 47, 1–16. [Google Scholar] [CrossRef]
- Lionnet, A.; Leclair-Visonneau, L.; Neunlist, M.; Murayama, S.; Takao, M.; Adler, C.H.; Derkinderen, P.; Beach, T.G. Does Parkinson’s disease start in the gut? Acta Neuropathol. 2018, 135, 1–12. [Google Scholar] [CrossRef]
- Garrido-Gil, P.; Rodriguez-Perez, A.I.; Dominguez-Meijide, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Bidirectional Neural Interaction Between Central Dopaminergic and Gut Lesions in Parkinson’s Disease Models. Mol. Neurobiol. 2018, 55, 7297–7316. [Google Scholar] [CrossRef]
- Iborra, S.F.; Vila, M.; Perier, C. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson’s Disease and Huntington’s Disease. Front. Neurosci. 2018, 12, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Chen, W.J.; Fu, R.H.; Tsai, C.W. Upregulation of OPA1 by carnosic acid is mediated through induction of IKKgamma ubiquitination by parkin and protects against neurotoxicity. Food Chem. Toxicol. 2020, 136, 110942. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.W.; Tsai, C.W. Carnosic acid protects SH-SY5Y cells against 6-hydroxydopamine-induced cell death through upregulation of parkin pathway. Neuropharmacology 2016, 110, 109–117. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.W. Carnosic Acid Attenuates 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Cells by Inducing Autophagy Through an Enhanced Interaction of Parkin and Beclin1. Mol. Neurobiol. 2017, 54, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, F.; Kim, T.E.; Bill, A.; Dettmer, U. Dynamic behaviors of alpha-synuclein and tau in the cellular context: New mechanistic insights and therapeutic opportunities in neurodegeneration. Neurobiol. Dis. 2019, 132, 104543. [Google Scholar] [CrossRef]
- Snyder, H.; Mensah, K.; Theisler, C.; Lee, J.; Matouschek, A.; Wolozin, B. Aggregated and monomeric alpha-synuclein bind to the S6’ proteasomal protein and inhibit proteasomal function. J. Biol. Chem. 2003, 278, 11753–11759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridi, J.C.; Hirth, F. Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Bayne, A.N.; Trempe, J.F. Mechanisms of PINK1, ubiquitin and Parkin interactions in mitochondrial quality control and beyond. Cell. Mol. Life Sci. 2019, 76, 4589–4611. [Google Scholar] [CrossRef]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giguere, N.; Pacelli, C.; Saumure, C.; Bourque, M.J.; Matheoud, D.; Levesque, D.; Slack, R.S.; Park, D.S.; Trudeau, L.E. Comparative analysis of Parkinson’s disease-associated genes in mice reveals altered survival and bioenergetics of Parkin-deficient dopamine neurons. J. Biol. Chem. 2018, 293, 9580–9593. [Google Scholar] [CrossRef] [Green Version]
- Cookson, M.R. Parkin’s substrates and the pathways leading to neuronal damage. Neuromolecular Med. 2003, 3, 1–13. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Chin, L.S. Parkin-mediated K63-linked polyubiquitination: A signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy 2008, 4, 85–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonskaya, I.; Hebron, M.L.; Desforges, N.M.; Franjie, A.; Moussa, C.E. Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol. Med. 2013, 5, 1247–1262. [Google Scholar] [CrossRef]
- Dachsel, J.C.; Lucking, C.B.; Deeg, S.; Schultz, E.; Lalowski, M.; Casademunt, E.; Corti, O.; Hampe, C.; Patenge, N.; Vaupel, K.; et al. Parkin interacts with the proteasome subunit alpha4. FEBS Lett. 2005, 579, 3913–3919. [Google Scholar] [CrossRef] [Green Version]
- Um, J.W.; Im, E.; Lee, H.J.; Min, B.; Yoo, L.; Yoo, J.; Lubbert, H.; Stichel-Gunkel, C.; Cho, H.S.; Yoon, J.B.; et al. Parkin directly modulates 26S proteasome activity. J. Neurosci. 2010, 30, 11805–11814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barodia, S.K.; Creed, R.B.; Goldberg, M.S. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res. Bull. 2017, 133, 51–59. [Google Scholar] [CrossRef]
- Ham, S.J.; Lee, D.; Yoo, H.; Jun, K.; Shin, H.; Chung, J. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc. Natl. Acad. Sci. USA 2020, 117, 4281–4291. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Diez-Fairen, M.; Kim, J.J.; Singleton, A.B. Genetics of Parkinson’s disease: An introspection of its journey towards precision medicine. Neurobiol. Dis. 2020, 137, 104782. [Google Scholar] [CrossRef]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.J.; Zhu, L.; et al. Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(-/-) mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fang, J.; Huang, L.; Wang, J.; Huang, X. Sophora flavescens Ait: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2015, 172, 10–29. [Google Scholar] [CrossRef]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef] [PubMed]
- Nariai, Y.; Mizuguchi, H.; Ogasawara, T.; Nagai, H.; Sasaki, Y.; Okamoto, Y.; Yoshimura, Y.; Kitamura, Y.; Nemoto, H.; Takeda, N.; et al. Disruption of Heat Shock Protein 90 (Hsp90)-Protein Kinase Cdelta (PKCdelta) Interaction by (-)-Maackiain Suppresses Histamine H1 Receptor Gene Transcription in HeLa Cells. J. Biol. Chem. 2015, 290, 27393–27402. [Google Scholar] [CrossRef] [Green Version]
- Aratanechemuge, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Komiya, T. Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004, 12, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, J.H.; Lee, C.; Jin, Q.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Inhibitory constituents of Sophora tonkinensis on nitric oxide production in RAW 264.7 macrophages. Bioorg. Med. Chem. Lett. 2015, 25, 960–962. [Google Scholar] [CrossRef]
- Martinez, B.A.; Caldwell, K.A.; Caldwell, G.A.C. elegans as a model system to accelerate discovery for Parkinson disease. Curr. Opin. Genet. Dev. 2017, 44, 102–109. [Google Scholar] [CrossRef]
- Brunetti, G.; Di Rosa, G.; Scuto, M.; Leri, M.; Stefani, M.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan Maintenance and Prevention of Parkinson’s-like Phenotypes with Hydroxytyrosol and Oleuropein Aglycone in C. elegans. Int. J. Mol. Sci. 2020, 21, 2588. [Google Scholar] [CrossRef] [Green Version]
- Anand, N.; Holcom, A.; Broussalian, M.; Schmidt, M.; Chinta, S.J.; Lithgow, G.J.; Andersen, J.K.; Chamoli, M. Dysregulated iron metabolism in C. elegans catp-6/ATP13A2 mutant impairs mitochondrial function. Neurobiol. Dis. 2020, 139, 104786. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hao, J.; Tian, D.; Wen, Y.; Zhao, P.; Chen, H.; Lv, Y.; Yang, X. Antidiabetic Activity of a Flavonoid-Rich Extract from Sophora davidii (Franch.) Skeels in KK-Ay Mice via Activation of AMP-Activated Protein Kinase. Front. Pharmacol. 2018, 9, 760. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Jankovic, J.; Guo, Y.; Xie, W.; Le, W. Small interfering RNA targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem. Biophys. Res. Commun. 2005, 337, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, C.; van Dyk, H.C.; Engelbrecht, L.; van der Westhuizen, F.H.; Kinnear, C.; Loos, B.; Bardien, S. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol. Neurobiol. 2017, 54, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.E.; Mount, M.P.; Safarpour, F.; Abdel-Messih, E.; Callaghan, S.; Mazerolle, C.; Kitada, T.; Slack, R.S.; Wallace, V.; Shen, J.; et al. Inactivation of Pink1 gene in vivo sensitizes dopamine-producing neurons to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and can be rescued by autosomal recessive Parkinson disease genes, Parkin or DJ-1. J. Biol. Chem. 2012, 287, 23162–23170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, A.; Kawarai, T.; Paitel, E.; Sanjo, N.; Maj, M.; Scheid, M.; Chen, F.; Gu, Y.; Hasegawa, H.; Salehi-Rad, S.; et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J. Biol. Chem. 2005, 280, 34025–34032. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; Chou, A.H.; Yeh, T.H.; Li, A.H.; Chen, Y.L.; Kuo, Y.L.; Tsai, S.R.; Yu, S.T. PINK1 mutants associated with recessive Parkinson’s disease are defective in inhibiting mitochondrial release of cytochrome c. Neurobiol. Dis. 2007, 28, 216–226. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef] [Green Version]
- Pandey, T.; Sammi, S.R.; Nooreen, Z.; Mishra, A.; Ahmad, A.; Bhatta, R.S.; Pandey, R. Anti-ageing and anti-Parkinsonian effects of natural flavonol, tambulin from Zanthoxyllum aramatum promotes longevity in Caenorhabditis elegans. Exp. Gerontol. 2019, 120, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Casacuberta, I.; Juarez-Flores, D.L.; Moren, C.; Garrabou, G. Bioenergetics and Autophagic Imbalance in Patients-Derived Cell Models of Parkinson Disease Supports Systemic Dysfunction in Neurodegeneration. Front. Neurosci. 2019, 13, 894. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Li, W.; Wang, H. TrxR2 overexpression alleviates inflammation-mediated neuronal death via reducing the oxidative stress and activating the Akt-Parkin pathway. Toxicol. Res. (Camb) 2019, 8, 641–653. [Google Scholar] [CrossRef]
- Um, J.W.; Park, H.J.; Song, J.; Jeon, I.; Lee, G.; Lee, P.H.; Chung, K.C. Formation of parkin aggregates and enhanced PINK1 accumulation during the pathogenesis of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2010, 393, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ren, Y.; Zhao, J.; Feng, J. Parkin protects human dopaminergic neuroblastoma cells against dopamine-induced apoptosis. Hum. Mol. Genet. 2004, 13, 1745–1754. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhou, H.Y.; Li, B.; Niu, G.Z.; Chen, S.D. Downregulation of parkin damages antioxidant defenses and enhances proteasome inhibition-induced toxicity in PC12 cells. J. Neuroimmune Pharmacol. 2007, 2, 276–283. [Google Scholar] [CrossRef]
- Wu, L.; Luo, N.; Zhao, H.R.; Gao, Q.; Lu, J.; Pan, Y.; Shi, J.P.; Tian, Y.Y.; Zhang, Y.D. Salubrinal protects against rotenone-induced SH-SY5Y cell death via ATF4-parkin pathway. Brain Res. 2014, 1549, 52–62. [Google Scholar] [CrossRef]
- Wang, C.; Ko, H.S.; Thomas, B.; Tsang, F.; Chew, K.C.; Tay, S.P.; Ho, M.W.; Lim, T.M.; Soong, T.W.; Pletnikova, O.; et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin’s protective function. Hum. Mol. Genet. 2005, 14, 3885–3897. [Google Scholar] [CrossRef]
- Higashi, Y.; Asanuma, M.; Miyazaki, I.; Hattori, N.; Mizuno, Y.; Ogawa, N. Parkin attenuates manganese-induced dopaminergic cell death. J. Neurochem. 2004, 89, 1490–1497. [Google Scholar] [CrossRef]
- Bian, M.; Liu, J.; Hong, X.; Yu, M.; Huang, Y.; Sheng, Z.; Fei, J.; Huang, F. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. PLoS ONE 2012, 7, e39953. [Google Scholar] [CrossRef]
- Liu, B.; Traini, R.; Killinger, B.; Schneider, B.; Moszczynska, A. Overexpression of parkin in the rat nigrostriatal dopamine system protects against methamphetamine neurotoxicity. Exp. Neurol. 2013, 247, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Deng, Y.; Zhang, J.; Han, H.; Zhao, M.; Li, Y.; Zhang, C.; Tian, J.; Bing, G.; Zhao, L. PINK1/Parkin-mediated mitophagy alleviates chlorpyrifos-induced apoptosis in SH-SY5Y cells. Toxicology 2015, 334, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, I.H.; Bouman, L.; Schlehe, J.S.; Schlierf, A.; Schramm, J.E.; Wegener, E.; Nakaso, K.; Culmsee, C.; Berninger, B.; Krappmann, D.; et al. Parkin mediates neuroprotection through activation of IkappaB kinase/nuclear factor-kappaB signaling. J. Neurosci. 2007, 27, 1868–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khasnavis, S.; Pahan, K. Cinnamon treatment upregulates neuroprotective proteins Parkin and DJ-1 and protects dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neuroimmune Pharmacol. 2014, 9, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonia Angeline, M.; Sarkar, A.; Anand, K.; Ambasta, R.K.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef]
- Li, R.; Chen, J. Salidroside Protects Dopaminergic Neurons by Enhancing PINK1/Parkin-Mediated Mitophagy. Oxid. Med. Cell. Longev. 2019, 2019, 9341018. [Google Scholar] [CrossRef]
- Zhi, Y.; Jin, Y.; Pan, L.; Zhang, A.; Liu, F. Schisandrin A ameliorates MPTP-induced Parkinson’s disease in a mouse model via regulation of brain autophagy. Arch. Pharm. Res. 2019, 42, 1012–1020. [Google Scholar] [CrossRef]
- Ren, Z.L.; Wang, C.D.; Wang, T.; Ding, H.; Zhou, M.; Yang, N.; Liu, Y.Y.; Chan, P. Ganoderma lucidum extract ameliorates MPTP-induced parkinsonism and protects dopaminergic neurons from oxidative stress via regulating mitochondrial function, autophagy, and apoptosis. Acta Pharmacol. Sin. 2019, 40, 441–450. [Google Scholar] [CrossRef]
- Bentea, E.; Verbruggen, L.; Massie, A. The Proteasome Inhibition Model of Parkinson’s Disease. J. Parkinsons Dis. 2017, 7, 31–63. [Google Scholar] [CrossRef] [Green Version]
- Miki, Y.; Tanji, K.; Mori, F.; Utsumi, J.; Sasaki, H.; Kakita, A.; Takahashi, H.; Wakabayashi, K. Autophagy mediators (FOXO1, SESN3 and TSC2) in Lewy body disease and aging. Neurosci. Lett. 2018, 684, 35–41. [Google Scholar] [CrossRef]
- Pramstaller, P.P.; Schlossmacher, M.G.; Jacques, T.S.; Scaravilli, F.; Eskelson, C.; Pepivani, I.; Hedrich, K.; Adel, S.; Gonzales-McNeal, M.; Hilker, R.; et al. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Ann. Neurol. 2005, 58, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Lonskaya, I.; Desforges, N.M.; Hebron, M.L.; Moussa, C.E. Ubiquitination increases parkin activity to promote autophagic alpha-synuclein clearance. PLoS ONE 2013, 8, e83914. [Google Scholar] [CrossRef] [PubMed]
- Lonskaya, I.; Hebron, M.L.; Algarzae, N.K.; Desforges, N.; Moussa, C.E. Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson’s disease. Neuroscience 2013, 232, 90–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, Q.; Zhang, L.; Wang, Q.; Yang, Z.; Liu, J.; Feng, L. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019, 150, 104538. [Google Scholar] [CrossRef]
- Vijayakumaran, S.; Nakamura, Y.; Henley, J.M.; Pountney, D.L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Mol. Cell. Neurosci. 2019, 101, 103416. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Singh, S. Updates on immunity and inflammation in Parkinson disease pathology. J. Neurosci. Res. 2018, 96, 379–390. [Google Scholar] [CrossRef]
- Yoo, H.; Kang, M.; Pyo, S.; Chae, H.S.; Ryu, K.H.; Kim, J.; Chin, Y.W. SKI3301, a purified herbal extract from Sophora tonkinensis, inhibited airway inflammation and bronchospasm in allergic asthma animal models in vivo. J. Ethnopharmacol. 2017, 206, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Han, K.; Tilve, S.; Wu, K.; Geller, H.M.; Sack, M.N. Parkin targets NOD2 to regulate astrocyte endoplasmic reticulum stress and inflammation. Glia 2018, 66, 2427–2437. [Google Scholar] [CrossRef]
- Kao, Y.C.; Wei, W.Y.; Tsai, K.J.; Wang, L.C. High Fat Diet Suppresses Peroxisome Proliferator-Activated Receptors and Reduces Dopaminergic Neurons in the Substantia Nigra. Int. J. Mol. Sci. 2019, 21, 207. [Google Scholar] [CrossRef] [Green Version]
- Tucci, M.L.; Harrington, A.J.; Caldwell, G.A.; Caldwell, K.A. Modeling dopamine neuron degeneration in Caenorhabditis elegans. Methods Mol. Biol. 2011, 793, 129–148. [Google Scholar]
- Voisine, C.; Varma, H.; Walker, N.; Bates, E.A.; Stockwell, B.R.; Hart, A.C. Identification of potential therapeutic drugs for huntington’s disease using Caenorhabditis elegans. PLoS ONE 2007, 2, e504. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Amaro, R.L.; Valentine, E.R.; Carretero, M.; LeBoeuf, S.E.; Rangaraju, S.; Broaddus, C.D.; Solis, G.M.; Williamson, J.R.; Petrascheck, M. Measuring Food Intake and Nutrient Absorption in Caenorhabditis elegans. Genetics 2015, 200, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Moreno, C.J.; Porta de la Riva, M.; Martinez-Lara, E.; Siles, E.; Canuelo, A. Tyrosol, a simple phenol from EVOO, targets multiple pathogenic mechanisms of neurodegeneration in a C. elegans model of Parkinson’s disease. Neurobiol. Aging 2019, 82, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Maulik, M.; Mitra, S.; Bult-Ito, A.; Taylor, B.E.; Vayndorf, E.M. Behavioral Phenotyping and Pathological Indicators of Parkinson’s Disease in C. elegans Models. Front. Genet. 2017, 8, 77. [Google Scholar] [CrossRef]
- Wilhelm, T.; Byrne, J.; Medina, R.; Kolundzic, E.; Geisinger, J.; Hajduskova, M.; Tursun, B.; Richly, H. Neuronal inhibition of the autophagy nucleation complex extends life span in post-reproductive C. elegans. Genes Dev. 2017, 31, 1561–1572. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.W.; Tsai, R.T.; Liu, S.P.; Chen, C.S.; Tsai, M.C.; Chien, S.H.; Hung, H.S.; Lin, S.Z.; Shyu, W.C.; Fu, R.H. Neuroprotective Effects of Betulin in Pharmacological and Transgenic Caenorhabditis elegans Models of Parkinson’s Disease. Cell Transplant. 2017, 26, 1903–1918. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.C.; Chang, C.H.; Liao, V.H. Anti-Parkinsonian effects of beta-amyrin are regulated via LGG-1 involved autophagy pathway in Caenorhabditis elegans. Phytomedicine 2017, 36, 118–125. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.W. PINK1/parkin-mediated mitophagy pathway is related to neuroprotection by carnosic acid in SH-SY5Y cells. Food Chem. Toxicol. 2019, 125, 430–437. [Google Scholar] [CrossRef]



















| Genes of C. elegans/Human | Primer Sequences (5′-3′) | (Start→End) Size (bp) |
|---|---|---|
| Lrk-1/LRRK1 | Forward: TTTCAACACCCAATCTCCAAC Reverse: TGATACTCGCTTGCCACAC | (1983→2092) 110 |
| Pdr-1/PRKN | Forward: TGCTCGTCAACCTCTGTTC Reverse: TCACTTTCTCCTTCCCATCAC | (376→601) 226 |
| Pink-1/PINK1 | Forward: GAGACGATACCGACAAACAC Reverse: GGCATTTCCTCCAAGACTAAC | (882→1158) 277 |
| Djr-1.1/PARK7 | Forward: CGGATTAGATGGAGCCGAAC Reverse: ATCAGCCCACCAGACTCTAC | (111→305) 195 |
| Djr-1.2/PARK7 | Forward: GCTTTGATCCTTTTGCCACC Reverse: CTGCCAGTTTGCTACATCC | (19→247) 229 |
| Vps-35/VPS35 | Forward: AACTCTGCTCAAAACTACTCAC Reverse: CCACAACCTTCTTCCCATTC | (1953→2146) 194 |
| Catp-6/ATP13A3 | Forward: TCACACCATACCAACCTCC Reverse: GTTTCCAAGAGTCTTCAGAACC | (3092→3336) 245 |
| Dnj-27/DNAJC10 | Forward: TCCACTTATTGCTCACATTGTC Reverse: TCCACCATCAACTCCACATC | (427→635) 209 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, R.-T.; Tsai, C.-W.; Liu, S.-P.; Gao, J.-X.; Kuo, Y.-H.; Chao, P.-M.; Hung, H.-S.; Shyu, W.-C.; Lin, S.-Z.; Fu, R.-H. Maackiain Ameliorates 6-Hydroxydopamine and SNCA Pathologies by Modulating the PINK1/Parkin Pathway in Models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y Cell Line. Int. J. Mol. Sci. 2020, 21, 4455. https://doi.org/10.3390/ijms21124455
Tsai R-T, Tsai C-W, Liu S-P, Gao J-X, Kuo Y-H, Chao P-M, Hung H-S, Shyu W-C, Lin S-Z, Fu R-H. Maackiain Ameliorates 6-Hydroxydopamine and SNCA Pathologies by Modulating the PINK1/Parkin Pathway in Models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y Cell Line. International Journal of Molecular Sciences. 2020; 21(12):4455. https://doi.org/10.3390/ijms21124455
Chicago/Turabian StyleTsai, Rong-Tzong, Chia-Wen Tsai, Shih-Ping Liu, Jia-Xin Gao, Yun-Hua Kuo, Pei-Min Chao, Huey-Shan Hung, Woei-Cherng Shyu, Shinn-Zong Lin, and Ru-Huei Fu. 2020. "Maackiain Ameliorates 6-Hydroxydopamine and SNCA Pathologies by Modulating the PINK1/Parkin Pathway in Models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y Cell Line" International Journal of Molecular Sciences 21, no. 12: 4455. https://doi.org/10.3390/ijms21124455
APA StyleTsai, R. -T., Tsai, C. -W., Liu, S. -P., Gao, J. -X., Kuo, Y. -H., Chao, P. -M., Hung, H. -S., Shyu, W. -C., Lin, S. -Z., & Fu, R. -H. (2020). Maackiain Ameliorates 6-Hydroxydopamine and SNCA Pathologies by Modulating the PINK1/Parkin Pathway in Models of Parkinson’s Disease in Caenorhabditis elegans and the SH-SY5Y Cell Line. International Journal of Molecular Sciences, 21(12), 4455. https://doi.org/10.3390/ijms21124455