Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration
Abstract
:1. Introduction
2. Results
2.1. Effects of δ-TT on MC3T3-E1 Cell Cycle
2.2. Effect of δ-TT on MC3T3-E1 and BMSC Cell Migration
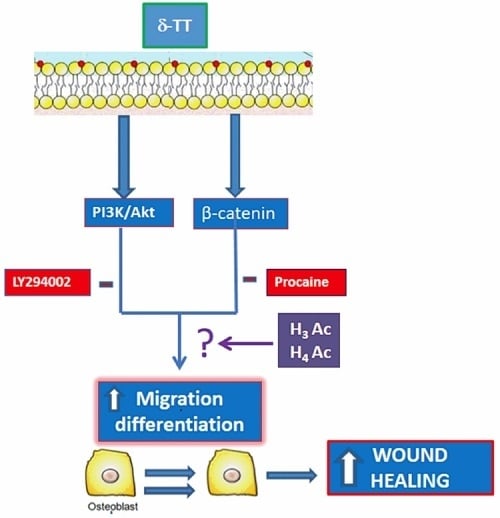
2.3. Involvement of Akt Pathway in δ-TT-Induced MC3T3-E1 Cell Migration
2.4. Beta-Catenin Is Involved in the Effect of δ-TT on Cell Migration
2.5. Epigenetic Mechanism: Effect of δ-TT on Histone Acetylation Levels
3. Discussion
4. Methods
4.1. δ-TT Purification
4.2. Cell Culture
4.3. Flow Cytometry Analysis
4.4. Scratch Wound Healing Assay
4.5. Morphological Studies
4.6. Boyden Chamber
4.7. Transcriptional Activity Analysis
4.8. Quantitative PCR Analysis
4.9. Alkaline Phosphatase (ALP) Activity and Collagen Content
4.10. Western Blotting Analysis
4.11. H3 and H4 Global Acetylation Levels
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Post, T.M.; Cremers, S.C.L.M.; Kerbusch, T.; Danhof, M. Bone Physiology, Disease and Treatment: Towards disease system analysis in osteoporosis. Clin. Pharmacokinet. 2010, 49, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H.; Roodman, D.; Freeman, C.; Mohla, S. Mechanisms of Tumor Metastasis to the Bone: Challenges and Opportunities. J. Bone Miner. Res. 2003, 18, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Boyde, A. The migration of osteoblasts. Cell Tissue Res. 1977, 184, 179–193. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Sarkar, M.; Chaubey, P.; Khan, T.; Sherje, A.P.; Patel, K.; Dravyakar, B. Bone Health and Natural Products- An Insight. Front. Pharmacol. 2018, 9, 981. [Google Scholar] [CrossRef]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Ali, H.M.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm Tocotrienol Supplementation Enhanced Bone Formation in Oestrogen-Deficient Rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Ding, Y.; Peng, Y.; Wu, Y.; Fan, J.; Li, W.; Yang, R.; Yang, M.; Fu, Q. γ-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone 2014, 67, 200–207. [Google Scholar] [CrossRef]
- Abdul-Majeed, S.; Mohamed, N.; Soelaiman, I.-N. Effects of Tocotrienol and Lovastatin Combination on Osteoblast and Osteoclast Activity in Estrogen-Deficient Osteoporosis. Evid.-Based Complement. Altern. Med. 2012, 2012, 960742. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.-Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.-Y. Effect of tocotrienol from Bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des. Dev. Ther. 2018, 12, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Moncaglieri, F.; Infantino, V.; Naso, M.; Perna, S. Focus on Pivotal Role of Dietary Intake (Diet and Supplement) and Blood Levels of Tocopherols and Tocotrienols in Obtaining Successful Aging. Int. J. Mol. Sci. 2015, 16, 23227–23249. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: A 12-week randomized double-blinded placebo-controlled trial. Osteoporos. Int. 2018, 29, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Abukhadir, S.S.A.; Mohamed, N.; Makpol, S.; Muhammad, N. Effects of Palm Vitamin E on Bone-Formation-Related Gene Expression in Nicotine-Treated Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 656025. [Google Scholar] [CrossRef]
- Hasan, W.N.W.; Ghafar, N.A.; Chin, K.-Y.; Ima-Nirwana, S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: A temporal sequential study. Drug Des. Dev. Ther. 2018, 12, 1715–1726. [Google Scholar] [CrossRef] [Green Version]
- Casati, L.; Pagani, F.; Limonta, P.; Vanetti, C.; Stancari, G.; Sibilia, V. Beneficial effects of delta-tocotrienol against oxidative stress in osteoblastic cells: Studies on the mechanisms of action. Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Macsai, C.E.; Foster, B.K.; Xian, C.J. Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J. Cell. Physiol. 2008, 215, 578–587. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. APC in Cell Migration. Adv. Exp. Med. Biol. 2009, 656, 30–40. [Google Scholar]
- Raut, N.; Wicks, S.M.; Lawal, T.O.; Mahady, G.B. Epigenetic regulation of bone remodeling by natural compounds. Pharmacol. Res. 2019, 147, 104350. [Google Scholar] [CrossRef]
- Jablons, D.; Gao, Z.; Xu, Z.; Hung, M.-S.; Lin, Y.-C.; Wang, T.; Gong, M.; Zhi, X.; You, L. Procaine and procainamide inhibit the Wnt canonical pathway by promoter demethylation of WIF-1 in lung cancer cells. Oncol. Rep. 2009, 22, 1479–1484. [Google Scholar] [CrossRef]
- Herencia, C.; Diaz-Tocados, J.M.; Jurado, L.; De Oca, A.M.; Rodríguez-Ortiz, M.E.; Martín-Alonso, C.; Martinez-Moreno, J.M.; Vergara, N.; Rodríguez, M.; Almadén, Y.; et al. Procaine Inhibits Osteo/Odontogenesis through Wnt/β-Catenin Inactivation. PLoS ONE 2016, 11, e0156788. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadjidakis, D.J.; Androulakis, I.I. Bone Remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Van Hul, M.; Maes, C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res. Part C Embryo Today: Rev. 2013, 99, 170–191. [Google Scholar] [CrossRef]
- Sudo, H.; Kodama, H.A.; Amagai, Y.; Yamamoto, S.; Kasai, S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J. Cell Boil. 1983, 96, 191–198. [Google Scholar] [CrossRef]
- Prescott, D.M. Regulation of cell reproduction. Cancer Res. 1968, 28, 1815–1820. [Google Scholar]
- Komori, T. Regulation of Osteoblast Differentiation by Runx2. Adv. Exp. Med. Biol. 2009, 658, 43–49. [Google Scholar]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Ito, H. Chemokines in mesenchymal stem cell therapy for bone repair: A novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod. Rheumatol. 2010, 21, 113–121. [Google Scholar] [CrossRef]
- Su, P.; Tian, Y.; Yang, C.; Ma, X.; Wang, X.; Pei, J.; Qian, A. Mesenchymal Stem Cell Migration during Bone Formation and Bone Diseases Therapy. Int. J. Mol. Sci. 2018, 19, 2343. [Google Scholar] [CrossRef] [Green Version]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Jamora, C.; Dasgupta, R.; Kocieniewski, P.; Fuchs, E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 2003, 422, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. The Effects of Tocotrienol on Bone Peptides in a Rat Model of Osteoporosis Induced by Metabolic Syndrome: The Possible Communication between Bone Cells. Int. J. Environ. Res. Public Heal. 2019, 16, 3313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunters, A.; Armstrong, V.J.; Zaman, G.; Kypta, R.; Kawano, Y.; Lanyon, L.E.; Price, J.S. Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J. Boil. Chem. 2009, 285, 8743–8758. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.; Frenkel, B. Glucocorticoids Inhibit the Transcriptional Activity of LEF/TCF in Differentiating Osteoblasts in a Glycogen Synthase Kinase-3β-dependent and -independent Manner. J. Boil. Chem. 2004, 280, 2388–2394. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Boil. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonnell, I.; Grigoriadis, A.E.; Lam, E.W.-F.; Price, J.S.; Sunters, A. A specific role for phosphoinositide 3-kinase and AKT in osteoblasts? Front. Endocrinol. 2012, 3, 88. [Google Scholar] [CrossRef] [Green Version]
- Sotobori, T.; Ueda, T.; Myoui, A.; Yoshioka, K.; Nakasaki, M.; Yoshikawa, H.; Itoh, K. Bone morphogenetic protein-2 promotes the haptotactic migration of murine osteoblastic and osteosarcoma cells by enhancing incorporation of integrin β1 into lipid rafts. Exp. Cell Res. 2006, 312, 3927–3938. [Google Scholar] [CrossRef]
- Gamell, C.; Osses, N.; Bartrons, R.; Camps, M.; Rosa, J.; Ventura, F.; Rückle, T. BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J. Cell Sci. 2008, 121, 3960–3970. [Google Scholar] [CrossRef] [Green Version]
- Chin, K.-Y.; Abdul-Majeed, S.; Mohamed, N.; Ima-Nirwana, S. The Effects of Tocotrienol and Lovastatin Co-Supplementation on Bone Dynamic Histomorphometry and Bone Morphogenetic Protein-2 Expression in Rats with Estrogen Deficiency. Nutrients 2017, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Husain, A.; Jeffries, M.A. Epigenetics and Bone Remodeling. Curr. Osteoporos. Rep. 2017, 15, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zheng, S.; Zheng, J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, J.; Licht, R.; Bongers, M.; Van Der Klundert, T.; Arends, R.; Van Blitterswijk, C. Inhibition of Histone Acetylation as a Tool in Bone Tissue Engineering. Tissue Eng. 2006, 12, 2927–2937. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Park, H.T.; Kim, Y.J.; Bae, Y.C.; Suh, K.T.; Jung, J.S. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J. Cell. Biochem. 2005, 96, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Marelli, M.M.; Marzagalli, M.; Moretti, R.M.; Beretta, G.; Casati, L.; Comitato, R.; Gravina, G.L.; Festuccia, C.; Limonta, P. Vitamin E δ-tocotrienol triggers endoplasmic reticulum stress-mediated apoptosis in human melanoma cells. Sci. Rep. 2016, 6, 30502. [Google Scholar] [CrossRef]
- Dieci, E.; Casati, L.; Pagani, F.; Celotti, F.; Sibilia, V. Acylated and unacylated ghrelin protect MC3T3-E1 cells against tert-butyl hydroperoxide-induced oxidative injury: Pharmacological characterization of ghrelin receptor and possible epigenetic involvement. Amino Acids 2014, 46, 1715–1725. [Google Scholar] [CrossRef]
- Niada, S.; Giannasi, C.; Ferreira, L.M.J.; Milani, A.; Arrigoni, E.; Brini, A.T. 17β-estradiol differently affects osteogenic differentiation of mesenchymal stem/stromal cells from adipose tissue and bone marrow. Differentiation 2016, 92, 291–297. [Google Scholar] [CrossRef]
- Planz, V.; Wang, J.; Windbergs, M. Establishment of a cell-based wound healing assay for bio-relevant testing of wound therapeutics. J. Pharmacol. Toxicol. Methods 2018, 89, 19–25. [Google Scholar] [CrossRef]
- Casati, L.; Celotti, F.; Negri-Cesi, P.; Sacchi, M.C.; Castano, P.; Colciago, A. Platelet derived growth factor (PDGF) contained in Platelet Rich Plasma (PRP) stimulates migration of osteoblasts by reorganizing actin cytoskeleton. Cell Adhes. Migr. 2014, 8, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Casati, L.; Sendra, R.; Poletti, A.; Negri-Cesi, P.; Celotti, F. Androgen receptor activation by polychlorinated biphenyls: Epigenetic effects mediated by the histone demethylase Jarid1b. Epigenetics 2013, 8, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.-L.; Liu, H.-Z.; Zhang, Z.-C.; Zhao, H.-L.; Zhao, H.; Huang, Y.; Yao, J.-H.; Sun, T.-S. The influence of MicroRNA-150 in Osteoblast Matrix Mineralization. J. Cell. Biochem. 2015, 116, 2970–2979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-K.; Yang, L.; Meng, G.-L.; Fan, J.; Chen, J.-Z.; He, Q.-Z.; Chen, S.; Fan, J.-Z.; Luo, Z.-J.; Liu, J. Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur. J. Pharmacol. 2012, 689, 31–37. [Google Scholar] [CrossRef]
- Casati, L.; Pagani, F.; Fibiani, M.; Scalzo, R.L.; Sibilia, V. Potential of delphinidin-3-rutinoside extracted from Solanum melongena L. as promoter of osteoblastic MC3T3-E1 function and antagonist of oxidative damage. Eur. J. Nutr. 2018, 58, 1019–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullberg-Reinert, H.; Jundt, G. In situ measurement of collagen synthesis by human bone cells with a Sirius Red-based colorimetric microassay: Effects of transforming growth factor β2 and ascorbic acid 2-phosphate. Histochem. Cell Boil. 1999, 112, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Casati, L.; Pagani, F.; Braga, P.C.; Scalzo, R.L.; Sibilia, V. Nasunin, a new player in the field of osteoblast protection against oxidative stress. J. Funct. Foods 2016, 23, 474–484. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casati, L.; Pagani, F.; Maggi, R.; Ferrucci, F.; Sibilia, V. Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration. Int. J. Mol. Sci. 2020, 21, 4661. https://doi.org/10.3390/ijms21134661
Casati L, Pagani F, Maggi R, Ferrucci F, Sibilia V. Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration. International Journal of Molecular Sciences. 2020; 21(13):4661. https://doi.org/10.3390/ijms21134661
Chicago/Turabian StyleCasati, Lavinia, Francesca Pagani, Roberto Maggi, Francesco Ferrucci, and Valeria Sibilia. 2020. "Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration" International Journal of Molecular Sciences 21, no. 13: 4661. https://doi.org/10.3390/ijms21134661
APA StyleCasati, L., Pagani, F., Maggi, R., Ferrucci, F., & Sibilia, V. (2020). Food for Bone: Evidence for a Role for Delta-Tocotrienol in the Physiological Control of Osteoblast Migration. International Journal of Molecular Sciences, 21(13), 4661. https://doi.org/10.3390/ijms21134661