A “Population Dynamics” Perspective on the Delayed Life-History Effects of Environmental Contaminations: An Illustration with a Preliminary Study of Cadmium Transgenerational Effects over Three Generations in the Crustacean Gammarus
Abstract
:1. Introduction
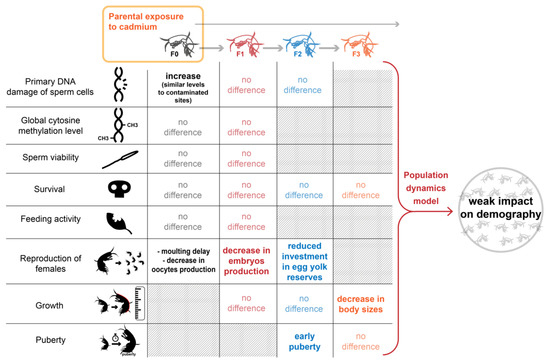
2. Results and Discussion
2.1. Experimental Design of the Transgenerational Study
2.2. Toxic Stress of Cd Exposure in F0 Spawners
2.3. Cascading Effects on F1, F2 Reproductive Features and F3 Growth Ability
2.4. Mitigation of the Demographic Impact along Generations
3. Materials and Methods
3.1. Test Protocol
3.2. Molecular and Physiological Measurements
3.3. Population Dynamics Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| KW | Kruskal–Wallis tests |
| MW | Mann–Whitney tests |
References
- Coutellec, M.-A.; Barata, C. Special issue on long-term ecotoxicological effects: An introduction. Ecotoxicology 2013, 22, 763–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckerman, A.; Benton, T.G.; Ranta, E.; Kaitala, V.; Lundberg, P. Population dynamic consequences of delayed life-history effects. Trends Ecol. Evol. 2002, 17, 263–269. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Cooke, S.J. Ecological carryover effects complicate conservation. Ambio 2015, 44, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammers-Wirtz, M.; Ratte, H.T. Offspring fitness in daphnia: Is the daphnia reproduction test appropriate for extrapolating effects on the population level? Environ. Toxicol. Chem. 2000, 19, 1856. [Google Scholar] [CrossRef]
- Castro, B.B.; Freches, A.R.; Rodrigues, M.; Nunes, B.; Antunes, S.C. Transgenerational Effects of Toxicants: An Extension of the Daphnia 21-day Chronic Assay? Arch. Environ. Contam. Toxicol. 2018, 74, 616–626. [Google Scholar] [CrossRef]
- Plautz, S.C.; Salice, C.J. Plasticity in offspring contaminant tolerance traits: Developmental cadmium exposure trumps parental effects. Ecotoxicology 2013, 22, 847–853. [Google Scholar] [CrossRef]
- Vigneron, A.; Geffard, O.; Quéau, H.; François, A.; Chaumot, A. Nongenetic inheritance of increased Cd tolerance in a field Gammarus fossarum population: Parental exposure steers offspring sensitivity. Aquat. Toxicol. 2019, 209, 91–98. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Guo, S.-N.; Yuan, S.-S.; Xia, H.; Zhu, Q.-L.; Lv, Z.-M. Preheating mitigates cadmium toxicity in zebrafish livers: Evidence from promoter demethylation, gene transcription to biochemical levels. Aquat. Toxicol. 2017, 190, 104–111. [Google Scholar] [CrossRef]
- Leblanc, G.A.; Wang, Y.H.; Holmes, C.N.; Kwon, G.; Medlock, E.K. A Transgenerational Endocrine Signaling Pathway in Crustacea. PLoS ONE 2013, 8, e61715. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.I.; Neher, D.A.; Ryoo, M.I. Life-history trade-offs of Paronychiurus kimi (Lee) (Collembola: Onychiuridae) populations exposed to paraquat. Ecotoxicol. Environ. Saf. 2008, 69, 227–232. [Google Scholar] [CrossRef]
- Prud’Homme, S.M.; Chaumot, A.; Cassar, E.; David, J.-P.; Reynaud, S. Impact of micropollutants on the life-history traits of the mosquito Aedes aegypti: On the relevance of transgenerational studies. Environ. Pollut. 2017, 220, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Villellas, J.; Doak, D.F.; Garcia, M.B.; Morris, W.F. Demographic compensation among populations: What is it, how does it arise and what are its implications? Ecol. Lett. 2015, 18, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, J.-M.; Yoccoz, N. Temporal variation in survival of mammals: A case of environmental canalization? Ecology 2003, 84, 3294–3306. [Google Scholar] [CrossRef]
- Raimondo, S.; Hemmer, B.L.; Goodman, L.R.; Cripe, G.M. Multigenerational exposure of the estuarine sheepshead minnow (Cyprinodon variegatus) to 17ß-Estradiol. II. Population-level effects through two life cycles. Environ. Toxicol. Chem. 2009, 28, 2409. [Google Scholar] [CrossRef]
- Biron, P.-A.; Massarin, S.; Alonzo, F.; Garcia-Sanchez, L.; Charles, S.; Billoir, E. Population-Level Modeling to Account for Multigenerational Effects of Uranium in Daphnia magna. Environ. Sci. Technol. 2011, 46, 1136–1143. [Google Scholar] [CrossRef]
- McKenney, C.L. The Influence of Insect Juvenile Hormone Agonists on Metamorphosis and Reproduction in Estuarine Crustaceans. Integr. Comp. Boil. 2005, 45, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Desneux, N.; Sonoda, S.; Liang, P.; Han, P.; Gao, X. Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop. Prot. 2013, 48, 29–34. [Google Scholar] [CrossRef]
- Piiroinen, S.; Boman, S.; Lyytinen, A.; Mappes, J.; Lindström, L. Sublethal effects of deltamethrin exposure of parental generations on physiological traits and overwintering in Leptinotarsa decemlineata. J. Appl. Èntomol. 2013, 138, 149–158. [Google Scholar] [CrossRef]
- Pakyari, H.; Enkegaard, A. Sublethal and Transgenerational Effects of Abamectin on the Biological Performance of the Predatory Thrips Scolothrips longicornis (Thysanoptera: Thripidae). J. Econ. Èntomol. 2015, 108, 559–565. [Google Scholar] [CrossRef]
- Parrott, J.L.; Bjerregaard, P.; Brugger, K.E.; Gray, L.E.; Iguchi, T.; Kadlec, S.M.; Weltje, L.; Wheeler, J.R. Uncertainties in biological responses that influence hazard and risk approaches to the regulation of endocrine active substances. Integr. Environ. Assess. Manag. 2017, 13, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Alfonso, S.; Blanc, M.; Joassard, L.; Keiter, S.; Munschy, C.; Loizeau, V.; Bégout, M.-L.; Cousin, X. Examining multi- and transgenerational behavioral and molecular alterations resulting from parental exposure to an environmental PCB and PBDE mixture. Aquat. Toxicol. 2019, 208, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, W.-X. Comparison between two clones of Daphnia magna: Effects of multigenerational cadmium exposure on toxicity, individual fitness, and biokinetics. Aquat. Toxicol. 2006, 76, 217–229. [Google Scholar] [CrossRef] [PubMed]
- De Liguoro, M.; Maraj, S.; Merlanti, R. Transgenerational toxicity of flumequine over four generations of Daphnia magna. Ecotoxicol. Environ. Saf. 2019, 169, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Nie, Y.; Liu, Y.; Dai, H.; Wang, J.; Si, B.; Yang, Z.; Cheng, L.; Liu, Y.; Chen, S.; et al. Transgenerational effects of diesel particulate matter on Caenorhabditis elegans through maternal and multigenerational exposure. Ecotoxicol. Environ. Saf. 2019, 170, 635–643. [Google Scholar] [CrossRef]
- Chaumot, A.; Geffard, O.; Armengaud, J.; Maltby, L. Gammarids as Reference Species for Freshwater Monitoring. In Aquatic Ecotoxicology; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 253–280. [Google Scholar]
- Ciliberti, A.; Chaumot, A.; Recoura-Massaquant, R.; Chandesris, A.; François, A.; Coquery, M.; Ferréol, M.; Geffard, O. Caged Gammarus as biomonitors identifying thresholds of toxic metal bioavailability that affect gammarid densities at the French national scale. Water Res. 2017, 118, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Alric, B.; Geffard, O.; Chandesris, A.; Ferreol, M.; François, A.; Perceval, O.; Piffady, J.; Villeneuve, B.; Chaumot, A. Multisubstance Indicators Based on Caged Gammarus Bioaccumulation Reveal the Influence of Chemical Contamination on Stream Macroinvertebrate Abundances across France. Environ. Sci. Technol. 2019, 53, 5906–5915. [Google Scholar] [CrossRef]
- Vigneron, A.; Geffard, O.; Coquery, M.; François, A.; Quéau, H.; Chaumot, A. Evolution of cadmium tolerance and associated costs in a Gammarus fossarum population inhabiting a low-level contaminated stream. Ecotoxicology 2015, 24, 1239–1249. [Google Scholar] [CrossRef]
- Cribiu, P.; Chaumot, A.; Geffard, O.; Ravanat, J.-L.; Bastide, T.; Delorme, N.; Quéau, H.; Caillat, S.; Devaux, A.; Bony, S. Natural variability and modulation by environmental stressors of global genomic cytosine methylation levels in a freshwater crustacean, Gammarus fossarum. Aquat. Toxicol. 2018, 205, 11–18. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, X.; Zhang, J.; Wang, R.; Yin, D. Transgenerational effects of heavy metals on L3 larva of Caenorhabditis elegans with greater behavior and growth inhibitions in the progeny. Ecotoxicol. Environ. Saf. 2013, 88, 178–184. [Google Scholar] [CrossRef]
- Kimberly, D.A.; Salice, C.J. Multigenerational contaminant exposures produce non-monotonic, transgenerational responses in Daphnia magna. Environ. Pollut. 2015, 207, 176–182. [Google Scholar] [CrossRef]
- Reátegui-Zirena, E.; Fidder, B.N.; Olson, A.D.; Dawson, D.E.; Bilbo, T.R.; Salice, C.J. Transgenerational endpoints provide increased sensitivity and insight into multigenerational responses of Lymnaea stagnalis exposed to cadmium. Environ. Pollut. 2017, 224, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Lacaze, E.; Geffard, O.; Goyet, D.; Bony, S.; Devaux, A. Linking genotoxic responses in Gammarus fossarum germ cells with reproduction impairment, using the Comet assay. Environ. Res. 2011, 111, 626–634. [Google Scholar] [CrossRef]
- Coulaud, R.; Geffard, O.; Coquillat, A.; Quéau, H.; Charles, S.; Chaumot, A. Ecological Modeling for the Extrapolation of Ecotoxicological Effects Measured during in Situ Assays in Gammarus. Environ. Sci. Technol. 2014, 48, 6428–6436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geffard, O.; Xuereb, B.; Chaumot, A.; Geffard, A.; Biagianti, S.; Noël, C.; Abbaci, K.; Garric, J.; Charmantier, G.; Charmantier-Daures, M. Ovarian cycle and embryonic development in Gammarus fossarum: Application for reproductive toxicity assessment. Environ. Toxicol. Chem. 2010, 29, 2249–2259. [Google Scholar] [CrossRef]
- Chaumot, A.; Coulaud, R.; Adam, O.; Quéau, H.; Lopes, C.; Geffard, O. In Situ Reproductive Bioassay with Caged Gammarus fossarum (Crustacea): Part 1—Gauging the Confounding Influence of Temperature and Water Hardness. Environ. Toxicol. Chem. 2020, 39, 667–677. [Google Scholar] [CrossRef]
- Lacaze, E.; Geffard, O.; Bony, S.; Devaux, A. Genotoxicity assessment in the amphipod Gammarus fossarum by use of the alkaline Comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 700, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Trapp, J.; Armengaud, J.; Pible, O.; Gaillard, J.-C.; Abbaci, K.; Habtoul, Y.; Chaumot, A.; Geffard, O. Proteomic Investigation of MaleGammarus fossarum, a Freshwater Crustacean, in Response to Endocrine Disruptors. J. Proteome Res. 2015, 14, 292–303. [Google Scholar] [CrossRef]
- Gouveia, D.; Chaumot, A.; Charnot, A.; Quéau, H.; Armengaud, J.; Almunia, C.; Salvador, A.; Geffard, O. Assessing the relevance of a multiplexed methodology for proteomic biomarker measurement in the invertebrate species Gammarus fossarum: A physiological and ecotoxicological study. Aquat. Toxicol. 2017, 190, 199–209. [Google Scholar] [CrossRef]
- Lopes, C.; Chaumot, A.; Xuereb, B.; Coulaud, R.; Jubeaux, G.; Quéau, H.; François, A.; Geffard, O. In situ reproductive bioassay with caged Gammarus fossarum (crustacea): Part 2—evaluating the relevance of using a molt cycle temperature—dependent model as reference to assess toxicity in freshwater monitoring. Environ. Toxicol. Chem. 2020, 39, 678–691. [Google Scholar] [CrossRef]
- Coulaud, R.; Geffard, O.; Vigneron, A.; Quéau, H.; François, A.; Chaumot, A. Linking feeding inhibition with reproductive impairment in Gammarus confirms the ecological relevance of feeding assays in environmental monitoring: Ecological relevance of feeding assay in Gammarus. Environ. Toxicol. Chem. 2015, 34, 1031–1038. [Google Scholar] [CrossRef]
- Lacaze, E.; Devaux, A.; Mons, R.; Bony, S.; Garric, J.; Geffard, A.; Geffard, O. DNA damage in caged Gammarus fossarum amphipods: A tool for freshwater genotoxicity assessment. Environ. Pollut. 2011, 159, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, D.A.; Salice, C.J. If you could turn back time: Understanding transgenerational latent effects of developmental exposure to contaminants. Environ. Pollut. 2014, 184, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Foran, C.M.; Peterson, B.N.; Benson, W.H. Influence of parental and developmental cadmium exposure on endocrine and reproductive function in Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 345–354. [Google Scholar] [CrossRef]
- Cazan, A.M.; Klerks, P.L. Evidence of maternal copper and cadmium transfer in two live-bearing fish species. Ecotoxicology 2014, 23, 1774–1783. [Google Scholar] [CrossRef]
- Luo, J.; Pei, S.; Jing, W.; Zou, E.; Wang, L. Cadmium inhibits molting of the freshwater crab Sinopotamon henanense by reducing the hemolymph ecdysteroid content and the activities of chitinase and N-acetyl-β-glucosaminidase in the epidermis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 169, 1–6. [Google Scholar] [CrossRef]
- Jaegers, J.; Gismondi, E. Gammarid exposure to pyriproxyfen and/or cadmium: What effects on the methylfarnesoate signalling pathway? Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Arambourou, H.; Decamps, A.; Quéau, H.; Dabrin, A.; Neuzeret, D.; Chaumot, A. Use of Gammarus fossarum (Amphipoda) embryo for toxicity testing: A case study with cadmium. Environ. Toxicol. Chem. 2017, 36, 2436–2443. [Google Scholar] [CrossRef]
- Au, W.T.D.; Lee, C.Y.; Chan, K.L.; Wu, R. Reproductive impairment of sea urchins upon chronic exposure to cadmium. Part I: Effects on gamete quality. Environ. Pollut. 2001, 111, 1–9. [Google Scholar] [CrossRef]
- Hudspith, M.; Reichelt-Brushett, A.; Harrison, P.L. Factors affecting the toxicity of trace metals to fertilization success in broadcast spawning marine invertebrates: A review. Aquat. Toxicol. 2017, 184, 1–13. [Google Scholar] [CrossRef]
- Driessnack, M.K.; Matthews, A.L.; Raine, J.C.; Niyogi, S. Interactive effects of chronic waterborne copper and cadmium exposure on tissue-specific metal accumulation and reproduction in fathead minnow (Pimephales promelas). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 165–173. [Google Scholar] [CrossRef]
- Sellin, M.K.; Eidem, T.M.; Kolok, A.S. Cadmium Exposures in Fathead Minnows: Are There Sex-Specific Differences in Mortality, Reproductive Success, and Cd Accumulation? Arch. Environ. Contam. Toxicol. 2007, 52, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Erraud, A.; Bonnard, M.; Geffard, O.; Coulaud, R.; Poret, A.; Duflot, A.; Forget-Leray, J.; Geffard, A.; Xuereb, B. Signification of DNA integrity in sperm of Palaemon serratus (Pennant 1777): Kinetic responses and reproduction impairment. Mar. Environ. Res. 2019, 144, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Carcinogenicity of metal compounds: Possible role of DNA repair inhibition. Toxicol. Lett. 1998, 102, 235–239. [Google Scholar] [CrossRef]
- Emmanouil, C.; Sheehan, T.; Chipman, J. Macromolecule oxidation and DNA repair in mussel (Mytilus edulis L.) gill following exposure to Cd and Cr(VI). Aquat. Toxicol. 2007, 82, 27–35. [Google Scholar] [CrossRef]
- Meng, J.; Wang, W.-X.; Li, L.; Yin, Q.; Zhang, G. Cadmium effects on DNA and protein metabolism in oyster (Crassostrea gigas) revealed by proteomic analyses. Sci. Rep. 2017, 7, 11716. [Google Scholar] [CrossRef]
- McBirney, M.; King, S.; Pappalardo, M.; Houser, E.; Unkefer, M.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Winchester, P.; Skinner, M.K. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS ONE 2017, 12, e0184306. [Google Scholar] [CrossRef] [PubMed]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Dioxin (TCDD) Induces Epigenetic Transgenerational Inheritance of Adult Onset Disease and Sperm Epimutations. PLoS ONE 2012, 7, e46249. [Google Scholar] [CrossRef]
- Rattan, S.; Brehm, E.; Gao, L.; A Flaws, J. Di(2-Ethylhexyl) Phthalate Exposure During Prenatal Development Causes Adverse Transgenerational Effects on Female Fertility in Mice. Toxicol. Sci. 2018, 163, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Po, B.H.; Chiu, J.M. Transgenerational impairments of reproduction and development of the marine invertebrate Crepidula onyx resulted from long-term dietary exposure of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Environ. Pollut. 2018, 235, 730–738. [Google Scholar] [CrossRef]
- Maltby, L.; Naylor, C. Preliminary Observations on the Ecological Relevance of the Gammarus ‘Scope for Growth’ Assay: Effect of Zinc on Reproduction. Funct. Ecol. 1990, 4, 393. [Google Scholar] [CrossRef]
- Minguez, L.; Ballandonne, C.; Rakotomalala, C.; Dubreule, C.; Kientz-Bouchart, V.; Halm-Lemeille, M.-P. Transgenerational Effects of Two Antidepressants (Sertraline and Venlafaxine) on Daphnia magna Life History Traits. Environ. Sci. Technol. 2015, 49, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Scanes, E.; Parker, L.M.; O’Connor, W.A.; Gibbs, M.C.; Ross, P.M. Copper and ocean acidification interact to lower maternal investment, but have little effect on adult physiology of the Sydney rock oyster Saccostrea glomerata. Aquat. Toxicol. 2018, 203, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Caswell, H. Matrix Population Models: Construction, Analysis and Interpretation, 2th ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Kammenga, J.E.; Van Koert, P.H.G.; Koeman, J.H.; Bakker, J. Fitness consequences of toxic stress evaluated within the context of phenotypic plasticity. Ecol. Appl. 1997, 7, 726–734. [Google Scholar] [CrossRef]
- Forrow, D.M.; Maltby, L. Toward a mechanistic understanding of contaminant-induced changes in detritus processing in streams: Direct and indirect effects on detritivore feeding. Environ. Toxicol. Chem. 2000, 19, 2100–2106. [Google Scholar] [CrossRef]
- Kelly, D.W.; Dick, J.T.; Montgomery, W.I. The functional role of Gammarus (Crustacea, Amphipoda): Shredders, predators, or both? Hydrobiologia 2002, 485, 199–203. [Google Scholar] [CrossRef]
- MacNeil, C.; Dick, J.T.A.; Elwood, R.W. The trophic ecology of freshwater gammarus spp. (crustacea: Amphipoda): Problems and perspectives concerning the functional feeding group concept. Boil. Rev. Camb. Philos. Soc. 1997, 72, 349–364. [Google Scholar] [CrossRef]
- Coulaud, R.; Geffard, O.; Xuereb, B.; Lacaze, E.; Quéau, H.; Garric, J.; Charles, S.; Chaumot, A. In situ feeding assay with Gammarus fossarum (Crustacea): Modelling the influence of confounding factors to improve water quality biomonitoring. Water Res. 2011, 45, 6417–6429. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cribiu, P.; Devaux, A.; Garnero, L.; Abbaci, K.; Bastide, T.; Delorme, N.; Quéau, H.; Degli Esposti, D.; Ravanat, J.-L.; Geffard, O.; et al. A “Population Dynamics” Perspective on the Delayed Life-History Effects of Environmental Contaminations: An Illustration with a Preliminary Study of Cadmium Transgenerational Effects over Three Generations in the Crustacean Gammarus. Int. J. Mol. Sci. 2020, 21, 4704. https://doi.org/10.3390/ijms21134704
Cribiu P, Devaux A, Garnero L, Abbaci K, Bastide T, Delorme N, Quéau H, Degli Esposti D, Ravanat J-L, Geffard O, et al. A “Population Dynamics” Perspective on the Delayed Life-History Effects of Environmental Contaminations: An Illustration with a Preliminary Study of Cadmium Transgenerational Effects over Three Generations in the Crustacean Gammarus. International Journal of Molecular Sciences. 2020; 21(13):4704. https://doi.org/10.3390/ijms21134704
Chicago/Turabian StyleCribiu, Pauline, Alain Devaux, Laura Garnero, Khédidja Abbaci, Thérèse Bastide, Nicolas Delorme, Hervé Quéau, Davide Degli Esposti, Jean-Luc Ravanat, Olivier Geffard, and et al. 2020. "A “Population Dynamics” Perspective on the Delayed Life-History Effects of Environmental Contaminations: An Illustration with a Preliminary Study of Cadmium Transgenerational Effects over Three Generations in the Crustacean Gammarus" International Journal of Molecular Sciences 21, no. 13: 4704. https://doi.org/10.3390/ijms21134704
APA StyleCribiu, P., Devaux, A., Garnero, L., Abbaci, K., Bastide, T., Delorme, N., Quéau, H., Degli Esposti, D., Ravanat, J.-L., Geffard, O., Bony, S., & Chaumot, A. (2020). A “Population Dynamics” Perspective on the Delayed Life-History Effects of Environmental Contaminations: An Illustration with a Preliminary Study of Cadmium Transgenerational Effects over Three Generations in the Crustacean Gammarus. International Journal of Molecular Sciences, 21(13), 4704. https://doi.org/10.3390/ijms21134704