A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice
Abstract
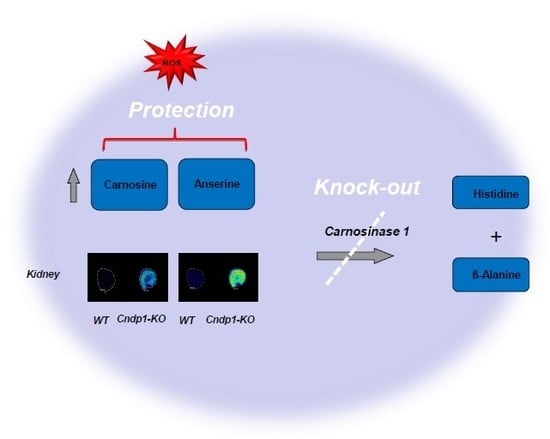
:1. Introduction
2. Material and Methods
2.1. Mouse Strains
2.2. Carnosinase Activity
2.3. Carnosine and Anserine Concentrations
2.4. Blood Glucose
2.5. Hematological Analysis
2.6. Food Intake
2.7. Localization of Carnosine, Anserine, GSH and GSSG
2.8. Histological Staining
2.9. Glomerular Filtration Rate
2.10. Albumin Creatinine Ratios
2.11. Intraperitoneal Insulin Tolerance Test (IPITT) and Intraperitoneal Glucose Tolerance Test (IPGTT)
2.12. Thiol- and Amino Acid Concentrations
2.13. Expression Analysis by qPCR
2.14. Protein Oxidation
2.15. Statistical Analysis
3. Results
3.1. Renal Carnosine and Anserine Metabolism
3.2. Non-Renal Tissue Carnosine and Anserine Metabolism
3.3. Amino Acid Profile
3.4. Kidney Morphology and Function
3.5. Oxidative Stress and Inflammation Marker
3.6. Glucose Homeostasis
3.7. Body and Organ Weight in Cndp1-KO and WT Mice
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Compliance with Ethical Standards
Abbreviations
| ACE | Angiotensin-converting enzyme |
| CEL | N-(1-Carboxyethyl)-L-lysine |
| CN1 | Carnosinase 1 |
| Cndp1 | Gene of murine carnosinase 1 |
| Cndp1-KO | Cndp1-knockout |
| FITC-sinistrin | fluorescein isothiocyanate-sinistrin |
| GAMM | Generalized additive mixed models |
| GFR | Glomerular filtration rate |
| GSH | reduced glutathione |
| GSSG | oxidized glutathione |
| HNE | 4-hydroxynonenal |
| HPLC | High performance liquid chromatography |
| Hspa1, Hspa1b | Genes of 70 kilodalton heat-shock protein |
| Hsf1 | heat shock factor 1 |
| IPGTT | Intraperitoneal glucose tolerance test |
| IPITT | intraperitoneal insulin tolerance test |
| MALDI | Matrix-assisted Laser Desorption/Ionization |
| MALDI-MSI | MALDI Mass Spectrometry Imaging |
| MG | Methylglyoxal |
| QTOF MS | quadrupole time-of-flight Mass Spectrometry |
| STZ | Streptozotocin |
| UPLC-FLR | ultra performance liquid chromatography-fluorescence detector |
| WT | wildtype |
References
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6251–6531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; Rychlik, I.; Cerna, M.; Romzova, M.; de Heer, E.; et al. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef] [Green Version]
- Mooyaart, A.L.; Zutinic, A.; Bakker, S.J.; Grootendorst, D.C.; Kleefstra, N.; van Valkengoed, I.G.; Bohringer, S.; Bilo, H.J.; Dekker, F.W.; Bruijn, J.A.; et al. Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes 2010, 59, 1555–1559. [Google Scholar] [CrossRef] [Green Version]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharm. 2008, 153, 6–20. [Google Scholar] [CrossRef] [Green Version]
- Vistoli, G.; Orioli, M.; Pedretti, A.; Regazzoni, L.; Canevotti, R.; Negrisoli, G.; Carini, M.; Aldini, G. Design, synthesis, and evaluation of carnosine derivatives as selective and efficient sequestering agents of cytotoxic reactive carbonyl species. Chem. Med. Chem. 2009, 4, 967–975. [Google Scholar] [CrossRef]
- Alhamdani, M.; Al-Azzawie, H.F.; Abbas, F.K. Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit Dial. Int. 2007, 27, 86–89. [Google Scholar] [CrossRef]
- Hou, W.; Chen, H.J.; Lin, Y.H. Antioxidant peptides with Angiotensin converting enzyme inhibitory activities and applications for Angiotensin converting enzyme purification. J. Agric. Food Chem. 2003, 51, 1706–1709. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ueno, A.; Nishikawa, Y. Interactions between carnosine and captopril on free radical scavenging activity and angiotensin-converting enzyme activity in vitro. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2006, 126, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Weigand, T.; Singler, B.; Fleming, T.; Nawroth, P.; Klika, K.D.; Thiel, C.; Baelde, H.; Garbade, S.F.; Wagner, A.H.; Hecker, M.; et al. Carnosine Catalyzes the Formation of the Oligo/Polymeric Products of Methylglyoxal. Cell Physiol. Biochem. 2018, 46, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; Colzani, M.; Mazzolari, A.; Gilardoni, E.; Rivaletto, C.; Carini, M.; Aldini, G. Quenching activity of carnosine derivatives towards reactive carbonyl species: Focus on alpha-(methylglyoxal) and beta-(malondialdehyde) dicarbonyls. Biochem. Biophys. Res. Commun. 2017, 492, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; De Buhr, S.; Beijer, B.; Lindner, T.; Wischnjow, A.; Kender, Z.; Peters, V.; Kopf, S.; Haberkorn, U.; et al. A scavenger peptide prevents methylglyoxal induced pain in mice. Biochim. Biophys. Acta 2017, 1863, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Mol, M.; Regazzoni, L.; Altomare, A.; Degani, G.; Carini, M.; Vistoli, G.; Aldini, G. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: Methodological aspects and biological consequences. Free Radic Biol. Med. 2017, 111, 328–344. [Google Scholar] [CrossRef]
- Decker, E.A.; Livisay, S.A.; Zhou, S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry 2000, 65, 766–770. [Google Scholar]
- Mozdzan, M.; Szemraj, J.; Rysz, J.; Nowak, D. Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin. Pharmacol. Toxicol. 2005, 96, 352–360. [Google Scholar] [CrossRef]
- Velez, S.; Nair, N.G.; Reddy, V.P. Transition metal ion binding studies of carnosine and histidine: Biologically relevant antioxidants. Colloids Surf. B Biointerfaces 2008, 66, 291–294. [Google Scholar] [CrossRef]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef] [Green Version]
- Peters, V.; Klessens, C.Q.; Baelde, H.J.; Singler, B.; Veraar, K.A.; Zutinic, A.; Drozak, J.; Zschocke, J.; Schmitt, C.P.; de Heer, E. Intrinsic carnosine metabolism in the human kidney. Amino ACIDS 2015. [Google Scholar] [CrossRef] [Green Version]
- Peters, V.; Yard, B.; Schmitt, C.P. Carnosine and diabetic nephropathy. Curr. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Peters, V.; Schmitt, C.P.; Zschocke, J.; Gross, M.L.; Brismar, K.; Forsberg, E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino ACIDS 2012, 42, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Ansurudeen, I.; Sunkari, V.G.; Grunler, J.; Peters, V.; Schmitt, C.P.; Catrina, S.B.; Brismar, K.; Forsberg, E.A. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino ACIDS 2012, 43, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Sauerhofer, S.; Yuan, G.; Braun, G.S.; Deinzer, M.; Neumaier, M.; Gretz, N.; Floege, J.; Kriz, W.; van der Woude, F.; Moeller, M.J. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 2007, 56, 2425–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, T.; Schilperoort, M.; Zhang, S.; Braun, J.D.; Qiu, J.; Rodriguez, A.; Pastene, D.O.; Kramer, B.K.; Koppel, H.; Baelde, H.; et al. Carnosine Attenuates the Development of both Type 2 Diabetes and Diabetic Nephropathy in BTBR ob/ob Mice. Sci. Rep. 2017, 7, 44492. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Blasetti Fantauzzi, C.; Pesce, C.M.; Giaccari, A.; Salomone, E.; Lapolla, A.; Orioli, M.; Aldini, G.; Pugliese, G. FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice. Br. J. Pharm. 2018, 175, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Menini, S.; Iacobini, C.; Ricci, C.; Scipioni, A.; Blasetti Fantauzzi, C.; Giaccari, A.; Salomone, E.; Canevotti, R.; Lapolla, A.; Orioli, M.; et al. D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br. J. Pharm. 2012, 166, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Baguet, A.; Everaert, I.; Yard, B.; Peters, V.; Zschocke, J.; Zutinic, A.; De Heer, E.; Podgorski, T.; Domaszewska, K.; Derave, W. Does low serum carnosinase activity favor high-intensity exercise capacity? J. Appl. Physiol. (Bethesda Md. 1985) 2014, 116, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Lexicon Pharmaceuticals, Inc. Available online: https://mmrrc.ucdavis.edu/phenotype/Genentech/PRT333N1/Expression/QC_Images/Level_I/popups/PRT333N1-Expression-QC_Images-imageViewer-1238-Southern.html (accessed on 3 July 2020).
- Schulz, S.; Becker, M.; Groseclose, M.R.; Schadt, S.; Hopf, C. Advanced MALDI mass spectrometry imaging in pharmaceutical research and drug development. Curr. Opin. Biotechnol. 2019, 55, 51–59. [Google Scholar] [CrossRef]
- Wirtz, M.; Droux, M.; Hell, R. O-acetylserine (thiol) lyase: An enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1785–1798. [Google Scholar] [CrossRef] [Green Version]
- Weger, B.D.; Weger, M.; Gorling, B.; Schink, A.; Gobet, C.; Keime, C.; Poschet, G.; Jost, B.; Krone, N.; Hell, R.; et al. Extensive Regulation of Diurnal Transcription and Metabolism by Glucocorticoids. PLoS Genet. 2016, 12, e1006512. [Google Scholar] [CrossRef]
- Riedl, E.; Pfister, F.; Braunagel, M.; Brinkkotter, P.; Sternik, P.; Deinzer, M.; Bakker, S.J.; Henning, R.H.; van den Born, J.; Kramer, B.K.; et al. Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol. Biochem. 2011, 28, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.C.; Chan, K.M.; Sharif, R. Antioxidant, Anti-inflammatory, and Genomic Stability Enhancement Effects of Zinc l-carnosine: A Potential Cancer Chemopreventive Agent? Nutr. Cancer 2017, 69, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Vistoli, G.; Stefek, M.; Chondrogianni, N.; Grune, T.; Sereikaite, J.; Sadowska-Bartosz, I.; Bartosz, G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013, 47 (Suppl. 1), 93–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Asp. Med. 2011, 32, 279–304. [Google Scholar] [CrossRef]
- Mikami, K.; Otaka, M.; Watanabe, D.; Goto, T.; Endoh, A.; Miura, K.; Ohshima, S.; Yoneyama, K.; Sato, M.; Shibuya, T.; et al. Zinc L-carnosine protects against mucosal injury in portal hypertensive gastropathy through induction of heat shock protein 72. J. Gastroenterol. Hepatol. 2006, 21, 1669–1674. [Google Scholar] [CrossRef]
- Nokin, M.J.; Durieux, F.; Peixoto, P.; Chiavarina, B.; Peulen, O.; Blomme, A.; Turtoi, A.; Costanza, B.; Smargiasso, N.; Baiwir, D.; et al. Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP-mediated tumor growth and metastasis. eLife 2016, 5. [Google Scholar] [CrossRef]
- Chebotareva, N.; Bobkova, I.; Shilov, E. Heat shock proteins and kidney disease: Perspectives of HSP therapy. Cell Stress Chaperones 2017, 22, 319–343. [Google Scholar] [CrossRef]
- Huynh, F.K.; Hu, X.; Lin, Z.; Johnson, J.D.; Hirschey, M.D. Loss of sirtuin 4 leads to elevated glucose- and leucine-stimulated insulin levels and accelerated age-induced insulin resistance in multiple murine genetic backgrounds. J. Inherit. Metab. Dis. 2018, 41, 59–72. [Google Scholar] [CrossRef]
- Peters, V.; Riedl, E.; Braunagel, M.; Hoger, S.; Hauske, S.; Pfister, F.; Zschocke, J.; Lanthaler, B.; Benck, U.; Hammes, H.P.; et al. Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul. Pept. 2014. [Google Scholar] [CrossRef]
- Nagai, K.; Tanida, M.; Niijima, A.; Tsuruoka, N.; Kiso, Y.; Horii, Y.; Shen, J.; Okumura, N. Role of L-carnosine in the control of blood glucose, blood pressure, thermogenesis, and lipolysis by autonomic nerves in rats: Involvement of the circadian clock and histamine. Amino ACIDS 2012, 43, 97–109. [Google Scholar] [CrossRef]
- Barca, A.; Gatti, F.; Spagnolo, D.; Ippati, S.; Vetrugno, C.; Verri, T. Responsiveness of Carnosine Homeostasis Genes in the Pancreas and Brain of Streptozotocin-Treated Mice Exposed to Dietary Carnosine. Int. J. Mol. Sci. 2018, 19, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Poll, M.C.; Soeters, P.B.; Deutz, N.E.; Fearon, K.C.; Dejong, C.H. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, A.A.; Karl, I.E.; Klahr, S. Role of arginine in health and in renal disease. Am. J. Physiol. 1994, 267, F331–F346. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Gao, C.; Hao, W.; Ji, C.; Zhao, L.; Zhang, J.; Liu, T.; Ma, Q. Effects of Dietary L-carnosine and Alpha-lipoic Acid on Growth Performance, Blood Thyroid Hormones and Lipid Profiles in Finishing Pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 1465–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmohl, F.; Peters, V.; Schmitt, C.P.; Poschet, G.; Buttner, M.; Li, X.; Weigand, T.; Poth, T.; Volk, N.; Morgenstern, J.; et al. CNDP1 knockout in zebrafish alters the amino acid metabolism, restrains weight gain, but does not protect from diabetic complications. Cell. Mol. Life Sci. CMLS 2019. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa-Kobayashi, K.; Otagi, E.; Ohhara, Y.; Goda, T.; Kasezawa, N.; Kayashima, Y. The Combined Effects of Genetic Variation in the CNDP1 and CNDP2 Genes and Dietary Carbohydrate and Carotene Intake on Obesity Risk. J. Nutr. Nutr. 2017, 10, 146–154. [Google Scholar] [CrossRef]
- Cripps, M.J.; Hanna, K.; Lavilla, C., Jr.; Sayers, S.R.; Caton, P.W.; Sims, C.; De Girolamo, L.; Sale, C.; Turner, M.D. Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake. Sci. Rep. 2017, 7, 13313. [Google Scholar] [CrossRef] [Green Version]









| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Hspa1a | TGGTGCAGTCCGACATGAAG | GCTGAGAGTCGTTGAAGTAGGC |
| Hspa1b | GAGATCGACTCTCTGTTCGAGG | GCCCGTTGAAGAAGTCCTG |
| Hsf1 | AACGTCCCGGCCTTCCTAA | AGATGAGCGCGTCTGTGTC |
| Hprt | TCAGTCAACGGGGGACATAAA | GGGGCTGTACTGCTTAACCAG |
| 11-Week-Old | 55-Week-Old | |||
|---|---|---|---|---|
| Wildtype | Both gender | Carnosine [nmol/mg] | 0.5 ± 0.9 | 0.2 ± 0.5 |
| Anserine [nmol/mg] | 1.0 ± 0.8 | 0.1 ± 0.04 c | ||
| Males | Carnosine [nmol/mg] | 0.9 ± 1.1 b | 0.4 ± 0.7 | |
| Anserine [nmol/mg] | 1.4 ± 1.1 | 0.1 ± 0.05 | ||
| Females | Carnosine [nmol/mg] | 0.3± 0.1 | 0.1 ± 0.09 c | |
| Anserine [nmol/mg] | 0.7 ± 0.4 | 0.1 ± 0.04 c | ||
| Cndp1-KO | Both gender | Carnosine [nmol/mg] | 1.9 ± 0.3 a | 0.4 ± 0.1 ac |
| Anserine [nmol/mg] | 2.4 ± 0.3 a | 0.9 ± 0.4 ac | ||
| Males | Carnosine [nmol/mg] | 1.7 ± 0.32 a | 0.7 ± 0.5 bc | |
| Anserine [nmol/mg] | 2.0 ± 0.6 a | 1.1 ± 0.3 ac | ||
| Females | Carnosine [nmol/mg] | 1.5 ± 0.3 a | 1.1 ± 0.3 a | |
| Anserine [nmol/mg] | 1.6 ± 0.7 a | 1.2 ± 0.3 a |
| 11-Week-Old Mice | Brain | Liver | Muscle | Heart | Lungs | ||
| Wildtype | Carnosine [nmol/mg] | 0.9 ± 0.3 | 0.2 ± 0.06 | 7.6 ± 1.7 | 0.3 ± 0.2 | 0.4 ± 0.4 | |
| Anserine [nmol/mg] | 0.3 ± 0.3 | 0.1 ± 0.04 | 8.2 ± 2.4 | 0.3 ± 0.2 | 0.2 ± 0.1 | ||
| Cndp1-KO | Carnosine [nmol/mg] | 0.7 ± 0.4 | 0.1 ± 0.08 * | 5.6 ± 0.7 * | 0.3 ± 0.1 | 0.3 ± 0.1 | |
| Anserine [nmol/mg] | 0.1 ± 0.05 | 0.04 ± 0.03 | 7.3 ± 1.5 | 0.1 ± 0.1 * | 0.1 ± 0.04 | ||
| 55-Week-Old Mice | Brain | Liver | Muscle | Heart | Lungs | Serum | |
| Wildtype | Carnosine [nmol/mg] | 1.4 ± 0.6 # | 0.1 ± 0.1 # | 8.5 ± 4.5 | 0.4 ± 0.6 | 0.7 ± 0.8 | 1.8 ± 0.8 |
| Anserine [nmol/mg] | 0.2 ± 0.1 | 0.1 ± 0.1 | 11.1 ± 5.2 | 1.4 ± 2.7 | 1.7 ± 2.1 # | 0.7 ± 0.3 | |
| Cndp1-KO | Carnosine [nmol/mg] | 2.3 ± 3.3 # | 0.1 ± 0.1 | 8.4 ± 4.9 | 0.5 ± 0.6 | 0.6 ± 0.8 | 1.7 ± 0.8 |
| Anserine [nmol/mg] | 0.1 ± 0.1 | 0.1 ± 0.1 # | 9.9 ± 5.2 | 1.0 ± 1.5 | 0.9 ± 1.6 | 1.5 ± 0.5 | |
| 11-Week-Old (g) | 55-Week-Old (g) | ||
|---|---|---|---|
| Wildtype | Males and Females | 22.5 ± 2.9 | 28.7 ± 5.5 |
| Males | 25.2 ± 1.3 | 32.3 ± 5.5 | |
| Females | 19.9 ± 0.6 | 26.5 ± 3.5 | |
| Cndp1-KO | Males and Females | 23.5 ± 3.8 | 33.8 ± 5 |
| Males | 26.7 ± 2.3 | 36.6 ± 3.9 | |
| Females | 20.2 ± 1.3 | 30.9 ± 4.2 | |
| Weeks of Life | Food Intake (g/Mouse/24 h) | |||
|---|---|---|---|---|
| WT | Number of Animals | Cndp1-KO | Number of Animals | |
| 20 | 2.90 ± 0.05 | 5 | 3.51± 0.05 | 6 |
| 24 | 3.05 ± 0.25 | 5 | 3.45 ± 0.05 | 6 |
| 28 | 3.14 ± 0.31 | 5 | 3.01 ± 0.05 | 6 |
| 32 | 2.81 ± 0.50 | 5 | 3.32 ± 0.05 | 6 |
| 36 | 2.99 ± 0.28 | 5 | 3.79 ± 0.05 | 6 |
| 40 | 3.12 ± 0.49 | 5 | 4.05 ± 0.05 | 3 |
| Mean | 3.00 | 3.36 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigand, T.; Colbatzky, F.; Pfeffer, T.; Garbade, S.F.; Klingbeil, K.; Colbatzky, F.; Becker, M.; Zemva, J.; Bulkescher, R.; Schürfeld, R.; et al. A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice. Int. J. Mol. Sci. 2020, 21, 4887. https://doi.org/10.3390/ijms21144887
Weigand T, Colbatzky F, Pfeffer T, Garbade SF, Klingbeil K, Colbatzky F, Becker M, Zemva J, Bulkescher R, Schürfeld R, et al. A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice. International Journal of Molecular Sciences. 2020; 21(14):4887. https://doi.org/10.3390/ijms21144887
Chicago/Turabian StyleWeigand, Tim, Florian Colbatzky, Tilman Pfeffer, Sven F. Garbade, Kristina Klingbeil, Florian Colbatzky, Michael Becker, Johanna Zemva, Ruben Bulkescher, Robin Schürfeld, and et al. 2020. "A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice" International Journal of Molecular Sciences 21, no. 14: 4887. https://doi.org/10.3390/ijms21144887
APA StyleWeigand, T., Colbatzky, F., Pfeffer, T., Garbade, S. F., Klingbeil, K., Colbatzky, F., Becker, M., Zemva, J., Bulkescher, R., Schürfeld, R., Thiel, C., Volk, N., Reuss, D., Hoffmann, G. F., Freichel, M., Hecker, M., Poth, T., Fleming, T., Poschet, G., ... Peters, V. (2020). A Global Cndp1-Knock-Out Selectively Increases Renal Carnosine and Anserine Concentrations in an Age- and Gender-Specific Manner in Mice. International Journal of Molecular Sciences, 21(14), 4887. https://doi.org/10.3390/ijms21144887