Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis
Abstract
:1. Introduction
1.1. Gut–Liver Axis and Dysbiosis
1.2. Sarcopenia and Liver Cirrhosis
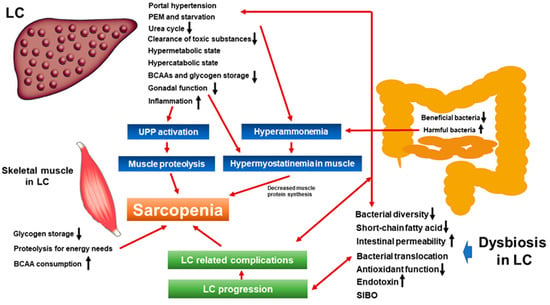
2. Liver Cirrhosis, Hepatic Encephalopathy, and Sarcopenia: Mechanisms and Clinical Impact
3. Dysbiosis and Sarcopenia from the Viewpoint of Nutrition and Metabolism
4. Dysbiosis, Intestinal Permeability, Tight Junction, and Sarcopenia
5. Surrogate Markers for the Severity of Dysbiosis in Liver Cirrhosis
6. Small Intestine Bacterial Overgrowth in Liver Cirrhosis
7. Dysbiosis and Bile Acid
8. Gut Microbiome in Patients with CLDs and Other Diseases
9. Antibiotics, Dysbiosis, Ammonia-Lowering Strategies, and Sarcopenia
10. Probiotics, Dysbiosis, Ammonia-Lowering Strategies, and Sarcopenia
11. Exercise and Gut Microbiota in Liver Cirrhosis
12. Closing Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GM | gut microbiota |
| CLD | chronic liver disease |
| LC | liver cirrhosis |
| PEM | protein-energy-malnutrition |
| BCAA | branched-chain amino acid |
| HCC | hepatocellular carcinoma |
| SBP | spontaneous bacterial peritonitis |
| HE | hepatic encephalopathy |
| ACLF | acute on chronic liver failure |
| JSH | Japanese Society of Hepatology |
| CT | computed tomography |
| HCV | hepatitis C virus |
| PNALT | persistent normal alanine aminotransferase |
| COVID-19 | Coronavirus disease-19 |
| PAMPs | pathogen-associated molecular patterns |
| LPS | lipopolysaccharide |
| TLR | toll like receptor |
| NASH | non-alcoholic steatohepatitis |
| NAFLD | non-alcoholic fatty liver disease |
| HR | hazard ratio |
| CI | confidence interval |
| PPI | proton pump inhibitor |
| mTORC1 | mammalian target of rapamycin complex1 |
| TNF-α | tumor necrosis factor-alpha |
| CRC | colorectal cancer |
| SCFAs | short-chain fatty acids |
| HVPG | hepatic venous pressure gradient |
| TJ | tight junction |
| SIBO | small intestine bacterial overgrowth |
| BAs | bile acids |
| DCA | deoxycholic acid |
| RCT | randomized controlled trial |
References
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut-Liver Axis and the Intersection With the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar]
- Cazorla, S.I.; Maldonado-Galdeano, C.; Weill, R.; De Paula, J.; Perdigón, G.D.V. Oral Administration of Probiotics Increases Paneth Cells and Intestinal Antimicrobial Activity. Front. Microbiol. 2018, 9, 736. [Google Scholar]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar]
- Solga, S.F.; Diehl, A.M. Gut Flora-Based Therapy in Liver Disease? The Liver Cares About the Gut. Hepatology 2004, 39, 1197–1200. [Google Scholar]
- Andoh, A. Physiological Role of Gut Microbiota for Maintaining Human Health. Digestion 2016, 93, 176–181. [Google Scholar]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 2037838. [Google Scholar]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut Microbiome and Liver Diseases. Gut 2016, 65, 2035–2044. [Google Scholar]
- Federico, A.; Dallio, M.; Caprio, G.G.; Ormando, V.M.; Loguercio, C. Gut Microbiota and the Liver. Minerva Gastroenterol. Dietol. 2017, 63, 385–398. [Google Scholar]
- Acharya, C.; Sahingur, S.E.; Bajaj, J.S. Microbiota, Cirrhosis, and the Emerging Oral-Gut-Liver Axis. JCI Insight 2017, 2, 94416. [Google Scholar]
- Woodhouse, C.A.; Patel, V.C.; Singanayagam, A.; Shawcross, D.L. The Gut Microbiome as a Therapeutic Target in the Pathogenesis and Treatment of Chronic Liver Disease. Aliment. Pharmacol. Ther. 2018, 47, 192–202. [Google Scholar]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar]
- Amrane, S.; Hocquart, M.; Afouda, P.; Kuete, E.; Pham, T.P.; Dione, N.; Ngom, I.I.; Valles, C.; Bachar, D.; Raoult, D.; et al. Metagenomic and Culturomic Analysis of Gut Microbiota Dysbiosis During Clostridium Difficile Infection. Sci. Rep. 2019, 9, 1–8. [Google Scholar]
- Halfvarson, J.; Brislawn, C.J.; Lamendella, R.; Vázquez-Baeza, Y.; Walters, W.A.; Bramer, L.M.; D’Amato, M.; Bonfiglio, F.; McDonald, D.; Gonzalez, A.; et al. Dynamics of the Human Gut Microbiome in Inflammatory Bowel Disease. Nat. Microbiol. 2017, 2, 1–7. [Google Scholar]
- Shen, F.; Zheng, R.D.; Sun, X.Q.; Ding, W.J.; Wang, X.Y.; Fan, J.G. Gut Microbiota Dysbiosis in Patients With Non-Alcoholic Fatty Liver Disease. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 375–381. [Google Scholar]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life Gut Microbiome and Egg Allergy. Allergy 2018, 73, 1515–1524. [Google Scholar]
- Dzidic, M.; Abrahamsson, T.R.; Artacho, A.; Björkstén, B.; Collado, M.C.; Mira, A.; Jenmalm, M.C. Aberrant IgA Responses to the Gut Microbiota During Infancy Precede Asthma and Allergy Development. J. Allergy Clin. Immunol. 2017, 139, 1017–1025. [Google Scholar]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar]
- Teng, F.; Klinger, C.N.; Felix, K.M.; Bradley, C.P.; Wu, E.; Tran, N.L.; Umesaki, Y.; Wu, H.J. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 2016, 44, 875–888. [Google Scholar]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a Novel Frailty Index to Predict Mortality in Patients With End-Stage Liver Disease. Hepatology 2017, 66, 564–574. [Google Scholar]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Combined Albumin-Bilirubin Grade and Skeletal Muscle Mass as a Predictor in Liver Cirrhosis. J. Clin. Med. 2019, 8, 782. [Google Scholar]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Health-Related Quality of Life in Chronic Liver Diseases: A Strong Impact of Hand Grip Strength. J. Clin. Med. 2018, 7, 553. [Google Scholar]
- Müller, M.J.; Loyal, S.; Schwarze, M.; Lobers, J.; Selberg, O.; Ringe, B.; Pichlmayr, R. Resting Energy Expenditure and Nutritional State in Patients With Liver Cirrhosis before and after Liver Transplantation. Clin. Nutr. 1994, 13, 145–152. [Google Scholar]
- Lai, J.C.; Rahimi, R.S.; Verna, E.C.; Kappus, M.R.; Dunn, M.A.; McAdams-DeMarco, M.; Haugen, C.E.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; et al. Frailty Associated With Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019, 156, 1675–1682. [Google Scholar]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar]
- Poh, H.O.; Amber, H.; Vera, C.M.; Khaled, D.; Ravi, B.; Susan, M.G.; Diana, R.M. Sarcopenia in Chronic Liver Disease: Impact on Outcomes. Liver Transpl. 2019, 25, 1422–1438. [Google Scholar]
- Bhanji, R.A.; Moctezuma-Velazquez, C.; Duarte-Rojo, A.; Ebadi, M.; Ghosh, S.; Rose, C.; Montano-Loza, A.J. Myosteatosis and Sarcopenia Are Associated With Hepatic Encephalopathy in Patients with Cirrhosis. Hepatol. Int. 2018, 12, 377–386. [Google Scholar]
- Hanai, T.; Shiraki, M.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Masahito, S. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: A sex-stratified analysis. Hepatol. Res. 2019, 49, 1414–1426. [Google Scholar]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Takata, R.; Hasegawa, K.; Nakano, C.; et al. Prognostic significance of low skeletal muscle mass compared with protein-energy malnutrition in liver cirrhosis. Hepatol. Res. 2017, 47, 1042–1052. [Google Scholar]
- Bunchorntavakul, C.; Reddy, K.R. Review article: Malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment. Pharmacol. Ther. 2020, 51, 64–77. [Google Scholar]
- Schneeweiss, B.; Graninger, W.; Ferenci, P.; Eichinger, S.; Grimm, G.; Schneider, B.; Laggner, A.N.; Lenz, K.; Kleinberger, G. Energy metabolism in patients with acute and chronic liver disease. Hepatology 1990, 11, 387–393. [Google Scholar]
- Nishikawa, H.; Osaki, Y. Clinical Significance of Therapy Using Branched-Chain Amino Acid Granules in Patients with Liver Cirrhosis and Hepatocellular Carcinoma. Hepatol. Res. 2014, 44, 149–158. [Google Scholar]
- Nardelli, S.; Gioia, S.; Faccioli, J.; Riggio, O.; Ridola, L. Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy. World J. Gastroenterol. 2019, 25, 5257–5265. [Google Scholar]
- Hiraoka, A.; Michitaka, K.; Kiguchi, D.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Tomida, H.; et al. Efficacy of Branched-Chain Amino Acid Supplementation and Walking Exercise for Preventing Sarcopenia in Patients with Liver Cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1416–1423. [Google Scholar]
- Kitajima, Y.; Takahashi, H.; Akiyama, T.; Murayama, K.; Iwane, S.; Kuwashiro, T.; Tanaka, K.; Kawazoe, S.; Ono, N.; Eguchi, T.; et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J. Gastroenterol. 2018, 53, 427–437. [Google Scholar]
- Namba, M.; Hiramatsu, A.; Aikata, H.; Kodama, K.; Uchikawa, S.; Ohya, K.; Morio, K.; Fujino, H.; Nakahara, T.; Murakami, E.; et al. Management of refractory ascites attenuates muscle mass reduction and improves survival in patients with decompensated cirrhosis. J. Gastroenterol. 2020, 55, 217–226. [Google Scholar]
- Hiraoka, A.; Aibiki, T.; Okudaira, T.; Toshimori, A.; Kawamura, T.; Nakahara, H.; Suga, Y.; Azemoto, N.; Miyata, H.; Miyamoto, Y.; et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: Computed tomography is useful for evaluation. J. Gastroenterol. 2015, 50, 1206–1213. [Google Scholar]
- Maurice, J.; Pinzani, M. The stratification of cirrhosis. Hepatol. Res. 2020, 50, 535–541. [Google Scholar]
- Nagamatsu, A.; Kawaguchi, T.; Hirota, K.; Koya, S.; Tomita, M.; Hashida, R.; Kida, Y.; Narao, H.; Manako, Y.; Tanaka, D.; et al. Slow walking speed overlapped with low handgrip strength in chronic liver disease patients with hepatocellular carcinoma. Hepatol. Res. 2019, 49, 1427–1440. [Google Scholar]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar]
- Arai, H.; Akishita, M.; Chen, L.K. Growing research on sarcopenia in Asia. Geriatr. Gerontol. Int. 2014, 14, 1–7. [Google Scholar]
- Alfonso, J.C.; Gülistan, B.; Jürgen, B.; Yves, B.; Olivier, B.; Tommy, C.; Cyrus, C.; Francesco, L.; Yves, R.; Avan, A.S.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019, 48, 16–31. [Google Scholar]
- Lai, J.C.; Covinsky, K.E.; McCulloch, C.E.; Feng, S. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients with Cirrhosis. Am. J. Gastroenterol. 2018, 113, 235–242. [Google Scholar]
- Bhanji, R.A.; Montano-Loza, A.J.; Watt, K.D. SARCOPENIA IN CIRRHOSIS: Looking beyond the skeletal muscle loss to see the systemic disease. Hepatology 2019, 70, 2193–2203. [Google Scholar]
- Fukui, H. Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far? Diseases 2019, 7, 58. [Google Scholar]
- Aquilio, E.; Spagnoli, R.; Riggio, D.; Seri, S. Effects of Zinc on Hepatic Ornithine Transcarbamylase (OTC) Activity. J. Trace. Elem. Electrolytes. Health Dis. 1993, 7, 240–241. [Google Scholar]
- Gangarao, D.; Dawid, K.; Bo-Jhih, G.; Avinash, K.; Samjhana, T.; Dharmvir, S.; Maria, H.; Srinivasan, D. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J. Hepatol. 2016, 65, 929–937. [Google Scholar]
- Katsanos, C.S.; Kobayashi, H.; Sheffield-Moore, M.; Aarsland, A.; Wolfe, R.R. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am. J. Physiol. Endocrinol. Metab. 2006, 291, 381–387. [Google Scholar]
- Lin, S.Y.; Wang, Y.Y.; Chuang, Y.H.; Chen, C.J. Skeletal Muscle Proteolysis Is Associated With Sympathetic Activation and TNF-α-ubiquitin-proteasome Pathway in Liver Cirrhotic Rats. J. Gastroenterol. Hepatol. 2016, 31, 890–896. [Google Scholar]
- Lin, S.Y.; Chen, W.Y.; Lee, F.Y.; Huang, C.J.; Sheu, W.H. Activation of Ubiquitin-Proteasome Pathway Is Involved in Skeletal Muscle Wasting in a Rat Model With Biliary Cirrhosis: Potential Role of TNF-alpha. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 493–501. [Google Scholar]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of Autophagy and the Ubiquitin-Proteasome System by the FoxO Transcriptional Network During Muscle Atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar]
- García, P.S.; Cabbabe, A.; Kambadur, R.; Nicholas, G.; Csete, M. Brief reports: Elevated myostatin levels in patients with liver disease: A potential contributor to skeletal muscle wasting. Anesth. Analg. 2010, 111, 707–709. [Google Scholar]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar]
- Dasarathy, S. Myostatin and Beyond in Cirrhosis: All Roads Lead to Sarcopenia. J. Cachexia Sarcopenia Muscle 2017, 8, 864–869. [Google Scholar]
- Zietz, B.; Lock, G.; Plach, B.; Drobnik, W.; Grossmann, J.; Schölmerich, J.; Straub, R.H. Dysfunction of the hypothalamic-pituitary- glandular axes and relation to Child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur. J. Gastroenterol. Hepatol. 2003, 15, 495–501. [Google Scholar]
- Sinclair, M.; Grossmann, M.; Hoermann, R.; Angus, P.W.; Gow, P.J. Testosterone Therapy Increases Muscle Mass in Men With Cirrhosis and Low Testosterone: A Randomised Controlled Trial. J. Hepatol. 2016, 65, 906–913. [Google Scholar]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachexia Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar]
- Hanai, T.; Shiraki, M.; Watanabe, S.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; Shimizu, M. Sarcopenia Predicts Minimal Hepatic Encephalopathy in Patients With Liver Cirrhosis. Hepatol Res. 2017, 47, 1359–1367. [Google Scholar]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Lin, H.Y.; Han, D.S. Is Sarcopenia Associated With Hepatic Encephalopathy in Liver Cirrhosis? A Systematic Review and Meta-Analysis. J. Formos. Med. Assoc. 2019, 118, 833–842. [Google Scholar]
- Tsai, C.F.; Chen, M.H.; Wang, Y.P.; Chu, C.J.; Huang, Y.H.; Lin, H.C.; Hou, M.C.; Lee, F.Y.; Su, T.P.; Lu, C.L. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology 2017, 152, 134–141. [Google Scholar]
- Bajaj, J.S.; Acharya, C.; Fagan, A.; White, M.B.; Gavis, E.; Heuman, D.M.; Hylemon, P.B.; Fuchs, M.; Puri, P.; Schubert, M.L.; et al. Proton Pump Inhibitor Initiation and Withdrawal Affects Gut Microbiota and Readmission Risk in Cirrhosis. Am. J. Gastroenterol. 2018, 113, 1177–1186. [Google Scholar]
- Evans, P.L.; McMillin, S.L.; Weyrauch, L.A.; Witczak, C.A. Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise Training. Nutrients 2019, 11, 2432. [Google Scholar]
- Consitt, L.A.; Dudley, C.; Saxena, G. Impact of Endurance and Resistance Training on Skeletal Muscle Glucose Metabolism in Older Adult. Nutrients 2019, 11, 2636. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar]
- Dagdeviren, S.; Jung, D.Y.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Inashima, K.; Tran, D.A.; Hu, X.; et al. IL-10 Prevents Aging-Associated Inflammation and Insulin Resistance in Skeletal Muscle. FASEB J. 2017, 31, 701–710. [Google Scholar]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The Gut Microbiota Influences Skeletal Muscle Mass and Function in Mice. Sci. Transl. Med. 2019, 11, 5662. [Google Scholar]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar]
- Liu, T.; Guo, Z.; Song, X.; Liu, L.; Dong, W.; Wang, S.; Xu, M.; Yang, C.; Wang, B.; Cao, H. High-fat diet-induced dysbiosis mediates MCP-1/CCR2 axis-dependent M2 macrophage polarization and promotes intestinal adenoma-adenocarcinoma sequence. J. Cell. Mol. Med. 2020, 24, 2648–2662. [Google Scholar]
- Shimizu, Y. Gut microbiota in common elderly diseases affecting activities of daily living. World J. Gastroenterol. 2018, 24, 4750–4758. [Google Scholar]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nur. 2010, 13, 715–721. [Google Scholar]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar]
- Duan, Y.H.; Zeng, L.M.; Li, F.N.; Kong, X.F.; Xu, K.; Guo, Q.P.; Wang, W.L.; Zhang, L.Y. β-hydroxy-β-methyl Butyrate Promotes Leucine Metabolism and Improves Muscle Fibre Composition in Growing Pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1328–1339. [Google Scholar]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate Alleviates High Fat Diet-Induced Obesity Through Activation of Adiponectin-Mediated Pathway and Stimulation of Mitochondrial Function in the Skeletal Muscle of Mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar]
- Besten, G.; Gerding, A.; van Dijk, T.H.; Ciapaite, J.; Bleeker, A.; van Eunen, K.; Havinga, R.; Groen, A.K.; Reijngoud, D.J.; Bakker, B.M. Protection against the Metabolic Syndrome by Guar Gum-Derived Short-Chain Fatty Acids Depends on Peroxisome Proliferator-Activated Receptor γ and Glucagon-Like Peptide-1. PLoS ONE 2015, 10, 0136364. [Google Scholar]
- Usami, M.; Miyoshi, M.; Kanbara, Y.; Aoyama, M.; Sakaki, H.; Shuno, K.; Hirata, K.; Takahashi, M.; Ueno, K.; Hamada, Y.; et al. Analysis of Fecal Microbiota, Organic Acids and Plasma Lipids in Hepatic Cancer Patients With or Without Liver Cirrhosis. Clin. Nutr. 2013, 32, 444–451. [Google Scholar]
- Juanola, O.; Ferrusquía-Acosta, J.; García-Villalba, R.; Zapater, P.; Magaz, M.; Marín, A.; Olivas, P.; Baiges, A.; Bellot, P.; Turon, F.; et al. Circulating Levels of Butyrate Are Inversely Related to Portal Hypertension, Endotoxemia, and Systemic Inflammation in Patients With Cirrhosis. FASEB J. 2019, 33, 11595–11605. [Google Scholar]
- Vince, A.; Killingley, M.; Wrong, O.M. Effect of Lactulose on Ammonia Production in a Fecal Incubation System. Gastroenterology 1978, 74, 544–549. [Google Scholar]
- Hernández, M.A.G.; Canfora, E.E.; Jocken, J.W.E.; Blaak, E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar]
- Fasano, A. Zonulin, Regulation of Tight Junctions, and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar]
- Fasano, A. Intestinal Permeability and Its Regulation by Zonulin: Diagnostic and Therapeutic Implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar]
- Biolato, M.; Manca, F.; Marrone, G.; Cefalo, C.; Racco, S.; Miggiano, G.A.; Valenza, V.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal Permeability After Mediterranean Diet and Low-Fat Diet in Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2019, 25, 509–520. [Google Scholar]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven Gut Vascular Barrier Disruption Is a Prerequisite for Non-Alcoholic Steatohepatitis Development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar]
- Fukui, H. Increased intestinal permeability and decreased barrier function: Does it really influence the risk inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar]
- Llovet, J.M.; Bartolí, R.; Planas, R.; Cabré, E.; Jimenez, M.; Urban, A.; Ojanguren, I.; Arnal, J.; Gassull, M.A. Bacterial translocation in cirrhotic rats. Its role in the development of spontaneous bacterial peritonitis. Gut 1994, 35, 1648–1652. [Google Scholar]
- Llovet, J.M.; Bartolí, R.; March, F.; Planas, R.; Viñado, B.; Cabré, E.; Arnal, J.; Coll, P.; Ausina, V.; Gassull, M.A. Translocated intestinal bacteria cause spontaneous bacterial peritonitis in cirrhotic rats: Molecular epidemiologic evidence. J. Hepatol. 1998, 28, 307–313. [Google Scholar]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; et al. Rapid Skeletal Muscle Wasting Predicts Worse Survival in Patients With Liver Cirrhosis. Hepatol. Res. 2016, 46, 743–751. [Google Scholar]
- Inoue, T.; Nakayama, J.; Moriya, K.; Kawaratani, H.; Momoda, R.; Ito, K.; Iio, E.; Nojiri, S.; Fujiwara, K.; Yoneda, M.; et al. Gut Dysbiosis Associated With Hepatitis C Virus Infection. Clin. Infect. Dis. 2018, 67, 869–877. [Google Scholar]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated With Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar]
- Bloemen, J.G.; Olde Damink, S.W.; Venema, K.; Buurman, W.A.; Jalan, R.; Dejong, C.H. Short Chain Fatty Acids Exchange: Is the Cirrhotic, Dysfunctional Liver Still Able to Clear Them? Clin. Nutr. 2010, 29, 365–369. [Google Scholar]
- Rainer, F.; Horvath, A.; Sandahl, T.D.; Leber, B.; Schmerboeck, B.; Blesl, A.; Groselj-Strele, A.; Stauber, R.E.; Fickert, P.; Stiegler, P.; et al. Soluble CD163 and Soluble Mannose Receptor Predict Survival and Decompensation in Patients With Liver Cirrhosis, and Correlate With Gut Permeability and Bacterial Translocation. Aliment. Pharmacol. Ther. 2018, 47, 657–664. [Google Scholar]
- Elswefy, S.E.; Abdallah, F.R.; Atteia, H.H.; Wahba, A.S.; Hasan, R.A. Inflammation, Oxidative Stress and Apoptosis Cascade Implications in Bisphenol A-induced Liver Fibrosis in Male Rats. Int. J. Exp. Pathol. 2016, 97, 369–379. [Google Scholar]
- Casati, M.; Ferri, E.; Azzolino, D.; Cesari, M.; Arosio, B. Gut Microbiota and Physical Frailty Through the Mediation of Sarcopenia. Exp. Gerontol. 2019, 124, 110639. [Google Scholar]
- Kaji, K.; Takaya, H.; Saikawa, S.; Furukawa, M.; Sato, S.; Kawaratani, H.; Kitade, M.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. Rifaximin Ameliorates Hepatic Encephalopathy and Endotoxemia Without Affecting the Gut Microbiome Diversity. World J. Gastroenterol. 2017, 23, 8355–8366. [Google Scholar]
- Bajaj, J.S.; Vargas, H.E.; Reddy, K.R.; Lai, J.C.; O’Leary, J.G.; Tandon, P.; Wong, F.; Mitrani, R.; White, M.B.; Kelly, M.; et al. Association Between Intestinal Microbiota Collected at Hospital Admission and Outcomes of Patients With Cirrhosis. Clin. Gastrotenterol. Hepatol. 2019, 17, 756–765. [Google Scholar]
- Bajaj, J.S.; Liu, E.J.; Kheradman, R.; Fagan, A.; Heuman, D.M.; White, M.; Gavis, E.A.; Hylemon, P.; Sikaroodi, M.; Gillevet, P.M. Fungal dysbiosis in cirrhosis. Gut 2018, 67, 1146–1154. [Google Scholar]
- Yao, J.; Chang, L.; Yuan, L.; Duan, Z. Nutrition Status and Small Intestinal Bacterial Overgrowth in Patients With Virus-Related Cirrhosis. Asia. Pac. J. Clin. Nutr. 2016, 25, 283–291. [Google Scholar]
- Pande, C.; Kumar, A.; Sarin, S.K. Small-intestinal Bacterial Overgrowth in Cirrhosis Is Related to the Severity of Liver Disease. Aliment. Pharmacol. Ther. 2009, 29, 1273–1281. [Google Scholar]
- Zhang, Y.; Feng, Y.; Cao, B.; Tian, Q. The Effect of Small Intestinal Bacterial Overgrowth on Minimal Hepatic Encephalopathy in Patients With Cirrhosis. Arch. Med. Sci. 2016, 12, 592–596. [Google Scholar]
- Bauer, T.M.; Steinbrückner, B.; Brinkmann, F.E.; Ditzen, A.K.; Schwacha, H.; Aponte, J.J.; Pelz, K.; Kist, M.; Blum, H.E. Small Intestinal Bacterial Overgrowth in Patients With Cirrhosis: Prevalence and Relation With Spontaneous Bacterial Peritonitis. Am. J. Gastroenterol. 2001, 96, 2962–2967. [Google Scholar]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar]
- Kuno, T.; Hirayama-Kurogi, M.; Ito, S.; Ohtsuki, S. Reduction in Hepatic Secondary Bile Acids Caused by Short-Term Antibiotic-Induced Dysbiosis Decreases Mouse Serum Glucose and Triglyceride Levels. Sci. Rep. 2018, 8, 1–15. [Google Scholar]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, 15–20. [Google Scholar]
- Sasaki, T.; Kuboyama, A.; Mita, M.; Murata, S.; Shimizu, M.; Inoue, J.; Mori, K.; Sato, R. The Exercise-Inducible Bile Acid Receptor Tgr5 Improves Skeletal Muscle Function in Mice. J. Biol. Chem. 2018, 293, 10322–10332. [Google Scholar]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Gut Microbiota, Cirrhosis, and Alcohol Regulate Bile Acid Metabolism in the Gut. Dig. Dis. 2015, 33, 338–345. [Google Scholar]
- Zeng, Y.; Chen, S.; Fu, Y.; Wu, W.; Chen, T.; Chen, J.; Yang, B.; Ou, Q. Gut Microbiota Dysbiosis in Patients With Hepatitis B Virus-Induced Chronic Liver Disease Covering Chronic Hepatitis, Liver Cirrhosis and Hepatocellular Carcinoma. J. Viral. Hepat. 2020, 27, 143–155. [Google Scholar]
- Simbrunner, B.; Mandorfer, M.; Trauner, M.; Reiberger, T. Gut-liver axis signaling in portal hypertension. World J. Gastroenterol. 2019, 25, 5897–5917. [Google Scholar]
- Yin, M.; Bradford, B.U.; Wheeler, M.D.; Uesugi, T.; Froh, M.; Goyert, S.M.; Thurman, R.G. Reduced early alcohol-induced liver injury in CD14-deficient mice. J. Immunol. 2001, 166, 4737–4742. [Google Scholar]
- Uesugi, T.; Froh, M.; Arteel, G.E.; Bradford, B.U.; Thurman, R.G. Toll-like receptor4 is involved in the mechanism of early alcohol induced liver injury in mice. Hepatology 2001, 34, 101–108. [Google Scholar]
- Imajo, K.; Fujita, K.; Yoneda, M.; Nozaki, Y.; Ogawa, Y.; Shinohara, Y.; Kato, S.; Mawatari, H.; Shibata, W.; Kitani, H.; et al. Hyperresponsivity to Low-Dose Endotoxin During Progression to Nonalcoholic Steatohepatitis Is Regulated by Leptin-Mediated Signaling. Cell. Metab. 2012, 16, 44–54. [Google Scholar]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related Changes in Gut Microbiota Composition From Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar]
- Flemer, B.; Lynch, D.B.; Brown, J.M.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and Non-Tumour-Associated Microbiota in Colorectal Cancer. Gut 2017, 66, 633–643. [Google Scholar]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020. [Google Scholar] [CrossRef]
- Singh, S.; Khan, A. Clinical Characteristics and Outcomes of COVID-19 Among Patients With Pre-Existing Liver Disease in United States: A Multi-Center Research Network Study. Gastroenterology 2020. [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut Microbiome Analysis as a Tool Towards Targeted Non-Invasive Biomarkers for Early Hepatocellular Carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar]
- Kim, S.; Covington, A.; Pamer, E.G. The Intestinal Microbiota: Antibiotics, Colonization Resistance, and Enteric Pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar]
- Descombe, J.J.; Dubourg, D.; Picard, M.; Palazzin, E. Pharmacokinetic study of rifaximin after oral administration in healthy volunteers. Int. J. Clin. Pharmacol. Res. 1994, 14, 51–56. [Google Scholar]
- Kang, S.H.; Lee, Y.B.; Lee, J.H.; Nam, J.Y.; Chang, Y.; Cho, H.; Yoo, J.J.; Cho, Y.Y.; Cho, E.J.; Yu, S.J.; et al. Rifaximin Treatment Is Associated With Reduced Risk of Cirrhotic Complications and Prolonged Overall Survival in Patients Experiencing Hepatic Encephalopathy. Aliment. Pharmacol. Ther. 2017, 46, 845–855. [Google Scholar]
- Mullen, K.D.; Sanyal, A.J.; Bass, N.M.; Poordad, F.F.; Sheikh, M.Y.; Frederick, R.T.; Bortey, E.; Forbes, W.P. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin. Gastroenterol. Hepatol. 2014, 12, 1390–1397. [Google Scholar]
- Gangarapu, V.; Ince, A.T.; Baysal, B.; Kayar, Y.; Kılıç, U.; Gök, Ö.; Uysal, Ö.; Şenturk, H. Efficacy of Rifaximin on Circulating Endotoxins and Cytokines in Patients With Nonalcoholic Fatty Liver Disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 840–845. [Google Scholar]
- Gatta, L.; Scarpignato, C. Systematic Review With Meta-Analysis: Rifaximin Is Effective and Safe for the Treatment of Small Intestine Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616. [Google Scholar]
- Dalal, R.; McGee, R.G.; Riordan, S.M.; Webster, A.C. Probiotics for people with hepatic encephalopathy. Cochrane Database. Syst. Rev. 2017. [Google Scholar] [CrossRef]
- Goel, A.; Rahim, U.; Nguyen, L.H.; Stave, C.; Nguyen, M.H. Systematic Review With Meta-Analysis: Rifaximin for the Prophylaxis of Spontaneous Bacterial Peritonitis. Aliment. Pharmacol. Ther. 2017, 46, 1029–1036. [Google Scholar]
- Flamm, S.L.; Mullen, K.D.; Heimanson, Z.; Sanyal, A.J. Rifaximin Has the Potential to Prevent Complications of Cirrhosis. Therap. Adv. Gastroenterol. 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Davuluri, G.; Silva, R.N.E.; Engelen, M.P.K.J.; Ten Have, G.A.M.; Prayson, R.; Deutz, N.E.P.; Dasarathy, S. Ammonia Lowering Reverses Sarcopenia of Cirrhosis by Restoring Skeletal Muscle Proteostasis. Hepatology 2017, 65, 2045–2058. [Google Scholar]
- Schulz, C.; Schütte, K.; Vilchez-Vargas, R.; Vasapolli, R.; Malfertheiner, P. Long-Term Effect of Rifaximin With and Without Lactulose on the Active Bacterial Assemblages in the Proximal Small Bowel and Faeces in Patients With Minimal Hepatic Encephalopathy. Dig. Dis. 2019, 37, 161–169. [Google Scholar]
- Kimer, N.; Pedersen, J.S.; Tavenier, J.; Christensen, J.E.; Busk, T.M.; Hobolth, L.; Krag, A.; Al-Soud, W.A.; Mortensen, M.S.; Sørensen, S.J.; et al. Rifaximin Has Minor Effects on Bacterial Composition, Inflammation, and Bacterial Translocation in Cirrhosis: A Randomized Trial. J. Gastroenterol. Hepatol. 2018, 33, 307–314. [Google Scholar]
- Butt, N.I.; Butt, U.I.; Kakar, A.A.T.K.; Malik, T.; Siddiqui, A.M. Is Lactulose Plus Rifaximin Better Than Lactulose Alone in the Management of Hepatic Encephalopathy? J. Coll. Physicians Surg. Pak. 2018, 28, 115–117. [Google Scholar]
- Mekky, M.A.; Riad, A.R.; Gaber, M.A.; Abdel-Malek, M.O.; Swifee, Y.M. Rifaximin Versus Metronidazole in Management of Acute Episode of Hepatic Encephalopathy: An Open Labeled Randomized Clinical Trial. Arab. J. Gastroenterol. 2018, 19, 76–79. [Google Scholar]
- Higuera-de-la-Tijera, F.; Servín-Caamaño, A.I.; Salas-Gordillo, F.; Pérez-Hernández, J.L.; Abdo-Francis, J.M.; Camacho-Aguilera, J.; Alla, S.N.; Jiménez-Ponce, F. Primary Prophylaxis to Prevent the Development of Hepatic Encephalopathy in Cirrhotic Patients With Acute Variceal Bleeding. Can. J. Gastroenterol. Hepatol. 2018, 2018, 3015891. [Google Scholar]
- Kimer, N.; Gudmann, N.S.; Pedersen, J.S.; Møller, S.; Nielsen, M.J.; Leeming, D.J.; Karsdal, M.A.; Møller, H.J.; Bendtsen, F.; Grønbæk, H. No Effect of Rifaximin on Soluble CD163, Mannose Receptor or Type III and IV Neoepitope Collagen Markers in Decompensated Cirrhosis: Results From a Randomized, Placebo Controlled Trial. PLoS ONE 2018, 13, 0203200. [Google Scholar]
- Goyal, O.; Sidhu, S.S.; Kishore, H. Minimal Hepatic Encephalopathy in Cirrhosis- How Long to Treat? Ann. Hepatol. 2017, 16, 115–122. [Google Scholar]
- Lauridsen, M.M.; Mikkelsen, S.; Svensson, T.; Holm, J.; Klüver, C.; Gram, J.; Vilstrup, H.; Schaffalitzky de Muckadell, O.B. The Continuous Reaction Time Test for Minimal Hepatic Encephalopathy Validated by a Randomized Controlled Multi-Modal intervention-A Pilot Study. PLoS ONE 2017, 12, 0185412. [Google Scholar]
- Lim, Y.L.; Kim, M.Y.; Jang, Y.O.; Baik, S.K.; Kwon, S.O. Rifaximin and Propranolol Combination Therapy Is More Effective Than Propranolol Monotherapy for the Reduction of Portal Pressure: An Open Randomized Controlled Pilot Study. Gut Liver 2017, 11, 702–710. [Google Scholar]
- Ibrahim, E.S.; Alsebaey, A.; Zaghla, H.; Moawad Abdelmageed, S.; Gameel, K.; Abdelsameea, E. Long-term Rifaximin Therapy as a Primary Prevention of Hepatorenal Syndrome. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1247–1250. [Google Scholar]
- Kimer, N.; Pedersen, J.S.; Busk, T.M.; Gluud, L.L.; Hobolth, L.; Krag, A.; Møller, S.; Bendtsen, F. Copenhagen Rifaximin (CoRif) Study Group. Rifaximin Has No Effect on Hemodynamics in Decompensated Cirrhosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Hepatology 2017, 65, 592–603. [Google Scholar]
- Elfert, A.; Abo Ali, L.; Soliman, S.; Ibrahim, S.; Abd-Elsalam, S. Randomized-controlled Trial of Rifaximin Versus Norfloxacin for Secondary Prophylaxis of Spontaneous Bacterial Peritonitis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1450–1454. [Google Scholar]
- Sidhu, S.S.; Goyal, O.; Parker, R.A.; Kishore, H.; Sood, A. Rifaximin vs. Lactulose in Treatment of Minimal Hepatic Encephalopathy. Liver Int. 2016, 36, 378–385. [Google Scholar]
- Assem, M.; Elsabaawy, M.; Abdelrashed, M.; Elemam, S.; Khodeer, S.; Hamed, W.; Abdelaziz, A.; El-Azab, G. Efficacy and Safety of Alternating Norfloxacin and Rifaximin as Primary Prophylaxis for Spontaneous Bacterial Peritonitis in Cirrhotic Ascites: A Prospective Randomized Open-Label Comparative Multicenter Study. Hepatol. Int. 2016, 10, 377–385. [Google Scholar]
- Zeng, X.; Tang, X.J.; Sheng, X.; Ni, W.; Xin, H.G.; Chen, W.Z.; Jiang, C.F.; Lin, Y.; Shi, J.; Shi, B.; et al. Does Low-Dose Rifaximin Ameliorate Endotoxemia in Patients With Liver Cirrhosis: A Prospective Study. J. Dig. Dis. 2015, 16, 665–674. [Google Scholar]
- Mostafa, T.; Badra, G.; Abdallah, M. The Efficacy and the Immunomodulatory Effect of Rifaximin in Prophylaxis of Spontaneous Bacterial Peritonitis in Cirrhotic Egyptian Patients. Turk. J. Gastroenterol. 2015, 26, 163–1639. [Google Scholar]
- Khokhar, N.; Qureshi, M.O.; Ahmad, S.; Ahmad, A.; Khan, H.H.; Shafqat, F.; Salih, M. Comparison of Once a Day Rifaximin to Twice a Day Dosage in the Prevention of Recurrence of Hepatic Encephalopathy in Patients with Chronic Liver Disease. J. Gastroenterol. Hepatol. 2015, 30, 1420–1422. [Google Scholar]
- Sharma, K.; Pant, S.; Misra, S.; Dwivedi, M.; Misra, A.; Narang, S.; Tewari, R.; Bhadoria, A.S. Effect of Rifaximin, Probiotics, and L-Ornithine L-Aspartate on Minimal Hepatic Encephalopathy: A Randomized Controlled Trial. Saudi. J. Gastroenterol. 2014, 20, 225–232. [Google Scholar]
- Ali, B.; Zaidi, Y.A.; Alam, A.; Anjum, H.S. Efficacy of Rifaximin in Prevention of Recurrence of Hepatic Encephalopathy in Patients With Cirrhosis of Liver. J. Coll. Physicians Surg. Pak. 2014, 24, 269–273. [Google Scholar]
- Sharma, B.C.; Sharma, P.; Lunia, M.K.; Srivastava, S.; Goyal, R.; Sarin, S.K. A Randomized, Double-Blind, Controlled Trial Comparing Rifaximin Plus Lactulose With Lactulose Alone in Treatment of Overt Hepatic Encephalopathy. Am. J. Gastroenterol. 2013, 108, 1458–1463. [Google Scholar]
- Kalambokis, G.N.; Mouzaki, A.; Rodi, M.; Tsianos, E.V. Rifaximin Improves Thrombocytopenia in Patients With Alcoholic Cirrhosis in Association With Reduction of Endotoxaemia. Liver Int. 2012, 32, 467–475. [Google Scholar]
- Sidhu, S.S.; Goyal, O.; Mishra, B.P.; Sood, A.; Chhina, R.S.; Soni, R.K. Rifaximin Improves Psychometric Performance and Health-Related Quality of Life in Patients With Minimal Hepatic Encephalopathy (The RIME Trial). Am. J. Gastroenterol. 2011, 106, 307–316. [Google Scholar]
- Bajaj, J.S.; Heuman, D.M.; Wade, J.B.; Gibson, D.P.; Saeian, K.; Wegelin, J.A.; Hafeezullah, M.; Bell, D.E.; Sterling, R.K.; Stravitz, R.T.; et al. Rifaximin Improves Driving Simulator Performance in a Randomized Trial of Patients With Minimal Hepatic Encephalopathy. Gastroenterology 2011, 140, 478–487. [Google Scholar]
- Sanyal, A.; Younossi, Z.M.; Bass, N.M.; Mullen, K.D.; Poordad, F.; Brown, R.S.; Vemuru, R.P.; Mazen Jamal, M.; Huang, S.; Merchant, K.; et al. Randomised Clinical Trial: Rifaximin Improves Health-Related Quality of Life in Cirrhotic Patients With Hepatic Encephalopathy-A Double-Blind Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2011, 34, 853–861. [Google Scholar]
- Bass, N.M.; Mullen, K.D.; Sanyal, A.; Poordad, F.; Neff, G.; Leevy, C.B.; Sigal, S.; Sheikh, M.Y.; Beavers, K.; Frederick, T.; et al. Rifaximin Treatment in Hepatic Encephalopathy. N. Engl. J. Med. 2010, 362, 1071–1081. [Google Scholar]
- Ohara, M.; Ogawa, K.; Suda, G.; Kimura, M.; Maehara, O.; Shimazaki, T.; Suzuki, K.; Nakamura, A.; Umemura, M.; Izumi, T.; et al. L-Carnitine Suppresses Loss of Skeletal Muscle Mass in Patients With Liver Cirrhosis. Hepatol. Commun. 2018, 2, 906–918. [Google Scholar]
- Hiramatsu, A.; Aikata, H.; Uchikawa, S.; Ohya, K.; Kodama, K.; Nishida, Y.; Daijo, K.; Osawa, M.; Teraoka, Y.; Honda, F.; et al. Levocarnitine Use Is Associated With Improvement in Sarcopenia in Patients With Liver Cirrhosis. Hepatol. Commun. 2019, 3, 348–355. [Google Scholar]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut-liver Axis, Cirrhosis and Portal Hypertension: The Chicken and the Egg. Hepatol. Int. 2018, 12, 24–33. [Google Scholar]
- Briskey, D.; Heritage, M.; Jaskowski, L.A.; Peake, J.; Gobe, G.; Subramaniam, V.N.; Crawford, D.; Campbell, C.; Vitetta, L. Probiotics Modify Tight-Junction Proteins in an Animal Model of Nonalcoholic Fatty Liver Disease. Therap. Adv. Gastroenterol. 2016, 9, 463–472. [Google Scholar]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium Strains and Galactooligosaccharides Improve Intestinal Barrier Function in Obese Adults but Show No Synergism When Used Together as Synbiotics. Microbiome 2018, 6, 121. [Google Scholar]
- Sharma, B.C.; Singh, J. Probiotics in management of hepatic encephalopathy. Metab. Brain Dis. 2016, 31, 1295–1301. [Google Scholar]
- Xia, X.; Chen, J.; Xia, J.; Wang, B.; Liu, H.; Yang, L.; Wang, Y.; Ling, Z. Role of Probiotics in the Treatment of Minimal Hepatic Encephalopathy in Patients With HBV-induced Liver Cirrhosis. J. Int. Med. Res. 2018, 46, 3596–3604. [Google Scholar]
- Horvath, A.; Leber, B.; Schmerboeck, B.; Tawdrous, M.; Zettel, G.; Hartl, A.; Madl, T.; Stryeck, S.; Fuchs, D.; Lemesch, S.; et al. Randomised Clinical Trial: The Effects of a Multispecies Probiotic vs. Placebo on Innate Immune Function, Bacterial Translocation and Gut Permeability in Patients With Cirrhosis. Aliment. Pharmacol. Ther. 2016, 44, 926–935. [Google Scholar]
- Dhiman, R.K.; Rana, B.; Agrawal, S.; Garg, A.; Chopra, M.; Thumburu, K.K.; Khattri, A.; Malhotra, S.; Duseja, A.; Chawla, Y.K. Probiotic VSL#3 Reduces Liver Disease Severity and Hospitalization in Patients With Cirrhosis: A Randomized, Controlled Trial. Gastroenterology 2014, 147, 1327–1337. [Google Scholar]
- Lunia, M.K.; Sharma, B.C.; Sharma, P.; Sachdeva, S.; Srivastava, S. Probiotics Prevent Hepatic Encephalopathy in Patients With Cirrhosis: A Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2014, 12, 1003–1008. [Google Scholar]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; Puri, P.; Sterling, R.K.; Luketic, V.; Stravitz, R.T.; Siddiqui, M.S.; Fuchs, M.; et al. Randomised Clinical Trial: Lactobacillus GG Modulates Gut Microbiome, Metabolome and Endotoxemia in Patients With Cirrhosis. Aliment. Pharmacol. Ther. 2014, 39, 1113–1125. [Google Scholar]
- Gupta, N.; Kumar, A.; Sharma, P.; Garg, V.; Sharma, B.C.; Sarin, S.K. Effects of the Adjunctive Probiotic VSL#3 on Portal Haemodynamics in Patients With Cirrhosis and Large Varices: A Randomized Trial. Liver Int. 2013, 33, 1148–1157. [Google Scholar]
- Jayakumar, S.; Carbonneau, M.; Hotte, N.; Befus, A.D.; St Laurent, C.; Owen, R.; McCarthy, M.; Madsen, K.; Bailey, R.J.; Ma, M.; et al. VSL#3® Probiotic Therapy Does Not Reduce Portal Pressures in Patients With Decompensated Cirrhosis. Liver Int. 2013, 33, 1470–1477. [Google Scholar]
- Agrawal, A.; Sharma, B.C.; Sharma, P.; Sarin, S.K. Secondary Prophylaxis of Hepatic Encephalopathy in Cirrhosis: An Open-Label, Randomized Controlled Trial of Lactulose, Probiotics, and No Therapy. Am. J. Gastroenterol. 2012, 107, 1043–1050. [Google Scholar]
- Pande, C.; Kumar, A.; Sarin, S.K. Addition of Probiotics to Norfloxacin Does Not Improve Efficacy in the Prevention of Spontaneous Bacterial Peritonitis: A Double-Blind Placebo-Controlled Randomized-Controlled Trial. Eur. J. Gastroenterol. Hepatol. 2012, 24, 831–839. [Google Scholar]
- Pereg, D.; Kotliroff, A.; Gadoth, N.; Hadary, R.; Lishner, M.; Kitay-Cohen, Y. Probiotics for Patients With Compensated Liver Cirrhosis: A Double-Blind Placebo-Controlled Study. Nutrition 2011, 27, 177–181. [Google Scholar]
- Mittal, V.V.; Sharma, B.C.; Sharma, P.; Sarin, S.K. A Randomized Controlled Trial Comparing Lactulose, Probiotics, and L-ornithine L-aspartate in Treatment of Minimal Hepatic Encephalopathy. Eur. J. Gastroenterol. Hepatol. 2011, 23, 725–732. [Google Scholar]
- Saji, S.; Kumar, S.; Thomas, V. A Randomized Double-Blind Placebo Controlled Trial of Probiotics in Minimal Hepatic Encephalopathy. Trop. Gastroenterol. 2011, 32, 128–132. [Google Scholar]
- Malaguarnera, M.; Gargante, M.P.; Malaguarnera, G.; Salmeri, M.; Mastrojeni, S.; Rampello, L.; Pennisi, G.; Li Volti, G.; Galvano, F. Bifidobacterium Combined With Fructo-Oligosaccharide Versus Lactulose in the Treatment of Patients With Hepatic Encephalopathy. Eur. J. Gastroenterol. Hepatol. 2010, 22, 199–206. [Google Scholar]
- Chen, L.H.; Huang, S.Y.; Huang, K.C.; Hsu, C.C.; Yang, K.C.; Li, L.A.; Chan, C.H.; Huang, H.Y. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging (Albany NY) 2019, 11, 756–770. [Google Scholar]
- Carbajo-Pescador, S.; Porras, D.; García-Mediavilla, M.V.; Martínez-Flórez, S.; Juarez-Fernández, M.; Cuevas, M.J.; Mauriz, J.L.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Beneficial Effects of Exercise on Gut Microbiota Functionality and Barrier Integrity, and Gut-Liver Crosstalk in an in vivo Model of Early Obesity and Non-Alcoholic Fatty Liver Disease. Dis. Model. Mech. 2019, 12, 039206. [Google Scholar]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and Associated Dietary Extremes Impact on Gut Microbial Diversity. Gut 2014, 63, 1913–1920. [Google Scholar]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar]
- Huber, Y.; Pfirrmann, D.; Gebhardt, I.; Labenz, C.; Gehrke, N.; Straub, B.K.; Ruckes, C.; Bantel, H.; Belda, E.; Clément, K.; et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment. Pharmacol. Ther. 2019, 50, 930–939. [Google Scholar]
- Macías-Rodríguez, R.U.; Ilarraza-Lomelí, H.; Ruiz-Margáin, A.; Ponce-de-León-Rosales, S.; Vargas-Vorácková, F.; García-Flores, O.; Torre, A.; Duarte-Rojo, A. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin. Transl. Gastroenterol. 2016, 7, 180. [Google Scholar]


| 1. Environmental Barrier | Gut microbiota |
| 2. Biological Barrier | Antimicrobial peptide |
| Immune cells | |
| 3. Physical Barrier | Mucus layer |
| Tight junction |
| Author (Year) | Treatment | Design | Target Patients | n | Primary Endpoint | Main Result |
|---|---|---|---|---|---|---|
| Schulz C, et al. (2019) [129] | Rifaximin 550 mg twice daily alone continuously for 3 months vs. rifaximin combined with lactulose 30–60 mL daily for 3 months | RCT | Decompensated LC with MHE | 5 | MHE improvement | Significant improvement of MHE in all patients. No statistically significant changes in the bacterial community profile at each time point. |
| Kimer N, et al. (2018) [130] | Rifaximin for 4 weeks vs. placebo | RCT | Decompensated LC | 54 | BT and inflammation | No impact on the inflammatory state and only minor effects on BT and intestinal bacterial composition |
| Nutt NI, et al. (2018) [131] | Lactulose vs. Lactulose+ rifaximin 550 mg twice daily | RCT | HE due to decompensated LC | 130 | HE | No significant difference on HE (p = 0.276). |
| Mekky MA, et al. (2018) [132] | Rifaximin vs. metronidazole | RCT | Decompensated LC with an acute episode of OHE | 120 | OHE improvement | OHE improvement: 46 patients (76.7%) in the metronidazole group vs. 45 (75%) in the rifaximin group (p = 0.412). |
| Higuera-de-la-Tijera F, et al. (2018) [133] | Lactulose vs. L-ornithine L-aspartate (LOLA) vs. rifaximin vs. placebo | RCT | Decompensated LC with variceal bleeding | 87 | HE development | Lactulose vs. placebo: 54.5% vs. 27.3%, p = 0.06 LOLA vs. placebo: 54.5% vs. 22.7%, p = 0.03 Rifaximin vs. placebo: 54.5% vs. 23.8%, p = 0.04. |
| Kimer N, et al. (2018) [134] | Rifaximin for 4 weeks vs. placebo | RCT | Decompensated LC | 54 | Macrophage markers s CD163, sMR | sCD163 and sMR were associated with liver disease severity. No effect of rifaximin on sCD163 and sMR. |
| Goyal O, et al. (2017) [135] | Rifaximin (1200 mg/day) vs. lactulose (30–120 mL/day) for 3 months | RCT | Decompensated LC with MHE | 112 | MHE reversal | MHE reversal at 3 months: 73.7% (42/57) in the rifaximin group and 69.1% (38/55) in the lactulose group (p = 0.677). |
| Lauridsen MM, et al. (2017) [136] | Lactulose plus BCAAs plus rifaximin vs. triple placebos for 3 months | RCT | Decompensated LC without clinically manifest HE | 44 | Continuous reaction test time (CRT) | ΔCRT: 0.50 ± 0.20 vs. 0.13 ± 0.12 (p = 0.06). |
| Lim YL, et al. (2017) [137] | Propranolol monotherapy vs. rifaximin and propranolol combination therapy | RCT | Decompensated LC | 64 | HVPG | HVPG response rates: 56.2% in the propranolol vs. 87.5% in the combination, (p = 0.034). |
| Ibrahim ES, et al. (2017) [138] | Rifaximin 550 mg twice daily for 12 weeks vs. placebo | RCT | Decompensated LC | 80 | HRS occurrence | HRS occurrence: 9 (22.5%) in the control group vs. 2 (5%) in the rifaximin group; p = 0.048. |
| Kimer N, et al. (2017) [139] | Rifaximin for 4 weeks vs. placebo | RCT | Decompensated LC | 54 | HVPG | No significant difference on HVPG (p = 0.94). |
| Elfert A, et al. (2016) [140] | Rifaximin 1200 mg daily vs. norfloxacin 400 mg daily for 6 months | RCT | Decompensated LC with a previous episode of SBP | 262 | Prevention of SBP | Recurrence rate of SBP: 3.88% in the rifaximin vs. 14.13% in the norfloxacin (p = 0.04) Mortality: 13.74% in the rifaximin vs. 24.43% in the norfloxacin (p = 0.044). |
| Sidhu, et al. (2016) [141] | Rifaximin 400 mg thrice a day vs. lactulose 30–120 mL/day | RCT | MHE due to decompensated LC | 112 | MHE improvement | MHE reversal at 3 months: 73.7% (42/57) in the rifaximin arm and 69.1% (38/55) in the lactulose arm (p > 0.05). |
| Assem M, et al. (2016) [142] | Alternating use of norfloxacin and rifaximin vs. norfloxacin or rifaximin alone | RCT | Decompensated LC | 334 | Primary prophylaxis of SBP | Primary prophylaxis of SBP: 74.7% vs. 56.4% vs. 68.3%, (p < 0.048). |
| Zeng X, et al. (2015) [143] | Low dose rifaximin (800 mg/day, 2 weeks) vs. high dose rifaximin (1200 mg/day, 2 weeks) vs. placebo | RCT | Decompensated LC | 43 | Endotoxemia | 1.1 ± 0.8 EU/mL in the low dose rifaximin (p < 0.05) 1.0 ± 0.8 EU/mL in the high dose rifaximin (p < 0.05) 2.5 ± 1.8 EU/mL in the control group. |
| Mostafa T, et al. (2015) [144] | Rifaximin vs. norfloxacin for 6 months | RCT | Decompensated LC | 70 | Inflammatory markers | No significant difference on TNF-α, IL-6, and IL-10. |
| Khokhar N, et al. (2015) [145] | Rifaximin 550 mg once a day vs. rifaximin 550 mg twice daily | RCT | Decompensated LC with at least one episode of HE | 306 | HE recurrence | Twenty-seven patients in rifaximin 550 mg once a day and 54 patients in rifaximin 550 mg twice daily with breakthrough episode of HE (p = 0.088). |
| Sharma K, et al. (2014) [146] | L-ornithine l-aspartate (LOLA) vs. rifaximin vs. probiotics vs. placebo for 2 months | RCT | Decompensated LC with MHE | 124 | MHE improvement | Critical flicker frequency scores and improvement in psychometric tests after treatment were significantly higher (p < 0.05) for LOLA, rifaximin, and probiotics as compared with placebo group. |
| Ali B, et al. (2014) [147] | Rifaximin 550 mg twice daily for 6 months vs. placebo | RCT | Decompensated LC with at least one episode of HE | 126 | HE recurrence | Free of hepatic encephalopathy during study period: 40 out of 63 patients in the placebo group and 35 patients out of 63 patients in the rifaximin group (p = 0.56). |
| Sharma BC, et al. (2013) [148] | Lactulose plus rifaximin 1200 mg/day vs. lactulose plus placebo | RCT | Decompensated LC with OHE | 120 | Complete reversal of HE | Forty-eight (76%) in lactulose plus rifaximin compared with 29 (50.8%) in lactulose plus placebo had complete reversal of HE (p < 0.004). |
| Kalambokis GN, et al. (2012) [149] | Rifaximin 1200 mg/day vs. no treatment | RCT | Alcoholic LC with thrombocytopenia | 23 | Thrombocytopenia | In the rifaximin group, platelet counts increased significantly (83,100 ± 9700 vs. 99,600 ± 11,200/μL; p = 0.006) with significant reductions in endotoxin (1.28 ± 0.41 vs. 2.54 ± 0.86 EU/mL; p = 0.005). |
| Sidhu SS, et al. (2011) [150] | Placebo vs. rifaximin (1200 mg/day) for 8 weeks | RCT | Decompensated LC with MHE | 94 | MHE improvement | Significantly more patients in the rifaximin group presented reversal of MHE (75.5% (37/49) vs. 20% (9/45) in the placebo group; p < 0.0001). |
| Bajaj JS, et al. (2011) [151] | Rifaximin 550 mg twice daily vs. placebo for 8 weeks | RCT | Decompensated LC with MHE and current drivers | 42 | Improvement in driving performance | Rifaximin group made significantly greater improvements than placebo group in avoiding total driving errors (76% vs. 31%; p = 0.013), speeding (81% vs. 33%; p = 0.005), and illegal turns (62% vs. 19%; p = 0.01). |
| Sanyal A, et al. (2011) [152] | Rifaximin 550 mg twice daily vs. placebo for 6 months | RCT | Decompensated LC with a documented history of recurrent HE | 219 | Chronic Liver Disease Questionnaire (CLDQ) score | The time-weighted averages of the overall CLDQ score and each domain score were significantly higher in the rifaximin group vs. placebo (p-values ranged from 0.0087 to 0.0436). |
| Bass NM, et al. (2010) [153] | Rifaximin 550 mg twice daily vs. placebo for 6 months | RCT | Decompensated LC with remission from HE | 299 | First breakthrough episode of HE | Rifaximin significantly reduced the risk of an episode of HE compared with placebo over 6 months (HR with rifaximin, 0.42; 95% CI, 0.28 to 0.64; p < 0.001). |
| Author (Year) | Treatment | Design | Target Patients | n | Primary Endpoint | Main Result |
|---|---|---|---|---|---|---|
| Xia X, et al. (2018) [160] | Probiotics (Clostridium butyricum combined with B. infantis) vs. no probiotics for 3 months | RCT | Decompensated HBV-LC without OHE | 67 | Cognitive function and quantitative assessment of predominant fecal bacteria | The cognition was significantly improved after probiotic treatment. The predominant bacteria (Clostridium cluster I and Bifidobacterium) were significantly enriched in the probiotics-treated group. |
| Horvath A, et al. (2016) [161] | Probiotics (eight different bacterial strains) vs. placebo for 6 months | RCT | Decompensated LC | 80 | The change in phagocytic capacity of neutrophils | A significant increase in neutrophil resting burst (2.6–3.2%, p = 0.0134) and neopterin levels (7.7–8.4 nmol/L, p = 0.001) in the probiotics group but not in the placebo group. |
| Dhiman RK, et al. (2014) [162] | A probiotic preparation (VSL#3, 9 × 10(11) bacteria) vs. placebo for 6 months | RCT | Decompensated LC who had recovered from an episode of HE | 130 | Development of breakthrough HE | Development of breakthrough HE: 34.8% in the probiotic group vs. 51.6% in the placebo group; HR, 0.65; 95% CI, 0.38-1.11; p = 0.12. |
| Lunia MK, et al. (2014) [163] | Probiotics (1 × 10(8) colony-forming units, 3 times daily) vs. control | RCT | Decompensated LC without OHE | 160 | The development of OHE | Seven subjects in the probiotics group and 14 controls developed OHE (p < 0.05; HR for controls vs. probiotic group, 2.1; 95% CI, 1.31–6.53). |
| Bajaj JS, et al. (2014) [164] | Probiotic Lactobacillus GG (LGG) vs. placebo for 8 weeks | RCT | Decompensated LC | 37 | Endotoxin, systemic inflammation and microbiome | Only in the LGG group, endotoxemia and TNF-α decreased, microbiome changed (reduced Enterobacteriaceae and increased Clostridiales Incertae Sedis XIV and Lachnospiraceae relative abundance). |
| Gupta N, et al. (2013) [165] | Propranolol plus placebo vs. propranolol plus antibiotics (norfloxacin 400 mg twice daily) vs. propranolol plus probiotic (VSL#3, 900 billion/day) | RCT | Decompensated LC with large esophageal varices without history of variceal bleeding | 94 | HVPG | The mean fall in HVPG was greater with either adjunctive probiotics (3.7 mmHg vs. 2.1 mmHg, p = 0.061) or adjunctive antibiotics (3.4 mmHg) than with propranolol alone. |
| Jayakumar S, et al. (2013) [166] | Probiotics (VSL#3) vs. placebo for 2 months | RCT | Decompensated LC with an HVPG 10 mmHg or more | 17 | HVPG | Median HVPG change from baseline -11.6% in the probiotics vs. +2.8% in the placebo (p > 0.05) |
| Agrawal A, et al. (2012) [167] | Lactulose vs. three capsules of probiotics vs. no therapy | RCT | Decompensated LC who had recoverd from HE | 235 | The development of OHE | The development of OHE: lactulose vs. probiotics, p = 0.349; probiotics vs. no therapy, p = 0.02; lactulose vs. no therapy, p = 0.001). |
| Pande C, et al. (2012) [168] | Norfloxacin 400 mg/day with probiotics capsules vs. norfloxacin with a placebo for 6 months | RCT | Decompensated LC who had either recovered from SBP or who were at a high risk for SBP | 110 | The occurrence of SBP | The frequencies of SBP were similar in the two groups. The cumulative probability of mortality was also similar. |
| Pereg D, et al. (2011) [169] | Probiotics vs. placebo for 6 months | RCT | Decompensated LC with at least one major complication of LC in the past | 36 | The effect on clinical and laboratory parameters | Probiotics was not associated with significant differences in either clinical or laboratory parameters between the two groups. |
| Mittal VV, et al. (2011) [170] | Lactulose vs. probiotics vs. L-ornithine L-aspartate (LOLA) vs. no therapy for 3 months | RCT | Decompensated LC with MHE | 322 | The improvement of MHE | The improvement of MHE: lactulose, 47.5%; probiotics, 35%; LOLA, 35%; no therapy, 10%. MHE improved significantly in all three treatment groups compared with no treatment (p = 0.006). |
| Saji S, et al. (2011) [171] | Probiotics vs. placebo | RCT | Decompensated LC with MHE | 43 | The improvement of MHE | There was no statistically significant change in the parameters (arterial ammonia, evoked responses and number connection test) between probiotics and placebo. |
| Malaguarnera M, et al. (2010) [172] | Bifidobacterium plus fructo-oligosaccharides (FOS) vs. lactulose for 2 months | RCT | Decompensated LC with HE | 125 | The improvement of HE | Bifidobacterium plus FOS-treated group compared with lactulose group showed a significant decrease of serum ammonia (p < 0.001), Trail Making Test A (p < 0.05) and B (p < 0.001), and a significant increase of Symbol Digit Modalities Test (p < 0.001) and Block Design Test (p < 0.001). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Enomoto, H.; Nishiguchi, S.; Iijima, H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int. J. Mol. Sci. 2020, 21, 5254. https://doi.org/10.3390/ijms21155254
Nishikawa H, Enomoto H, Nishiguchi S, Iijima H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. International Journal of Molecular Sciences. 2020; 21(15):5254. https://doi.org/10.3390/ijms21155254
Chicago/Turabian StyleNishikawa, Hiroki, Hirayuki Enomoto, Shuhei Nishiguchi, and Hiroko Iijima. 2020. "Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis" International Journal of Molecular Sciences 21, no. 15: 5254. https://doi.org/10.3390/ijms21155254
APA StyleNishikawa, H., Enomoto, H., Nishiguchi, S., & Iijima, H. (2020). Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. International Journal of Molecular Sciences, 21(15), 5254. https://doi.org/10.3390/ijms21155254