Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects
Abstract
:1. Introduction
2. Aromatase Inhibitors: Development and Pharmacology
3. Safety and Tolerance Issues of AIs Therapy in BC
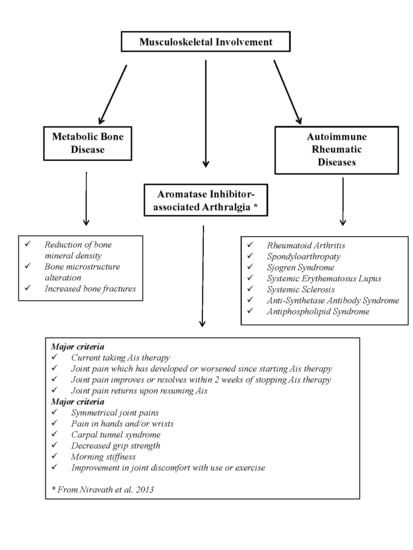
4. Musculoskeletal Disorders
4.1. AIs and Bone Health
4.1.1. Etiopathophysiology of AIs-Induced Bone Loss
4.1.2. Management of Bone Health in AIs-Treated Women
4.2. AI-Associated Arthralgia (AIA)
4.2.1. Etiopathophysiology of AI-Associated Arthralgia
4.2.2. Management of AI-Associated Arthralgia in AIs-Treated Women
Pharmacological Management of AI-Associated Arthralgia in AIs-Treated Women
Non-Pharmacological Management of AI-Associated Arthralgia in AIs-Treated Women
4.3. Rheumatic Autoimmune Diseases
4.3.1. Literature Data on the Association between AIs and Autoimmune Rheumatic Diseases
4.3.2. Literature Data on the Incidence of Rheumatic Diseases during BC Hormone Therapy
4.3.3. Etiopathophysiology of AIs-Induced Rheumatic Autoimmune Diseases
5. Conclusive Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACOG | American College of Obstetricians and Gynecologists |
| ACR | American College of Rheumatology |
| AIA | Aromatase inhibitors-associated arthralgia |
| AIs | Aromatase inhibitors |
| Anti-CCP | anti-cyclic citrullinated protein antibodies |
| aPL | anti-phospholipid antibodies |
| APS | Antiphospholipid syndrome |
| ArKO | Aromatase gene knockout |
| ASAS | Anti-synthetase antibody syndrome |
| ASCO | American Society of Clinical Oncology |
| BC | Breast cancer |
| Bcl-2 protein | B cell lymphoma-2 protein |
| BMD | Bone mineral density |
| BMI | Body mass index |
| BPI-SF | Brief pain inventory—short form |
| CDK | Cycline dependent kynase |
| CRP | C reactive protein |
| CTLs | Cytotoxic-T-lymphocytes |
| CV | Cardiovascular |
| DAS | Disease activity score |
| DASH | Disabilities of the arm, shoulder and hand |
| DLE | Discoid lupus erythematosus |
| DXA | Dual-energy X-ray absorptiometry |
| E1 | Estrone |
| E2 | Estradiol |
| ELPh | Exemestane and letrozole pharmacogenetics |
| ER | Estrogen receptors |
| ESMO | European Society of Medical Oncology |
| ESR | Erythrocyte sedimentation rate |
| FACT-ES TOI | Functional Assessment of Cancer Therapy-Endocrine Scale Trial Outcome Index |
| FDA | Food and Drug Administration |
| GSM | Genitourinary syndrome of menopause |
| GWAS | Genome-Wide Association Study |
| HAQII | Health Assessment Questionnaire II |
| HLA | Human leucocyte antigen |
| HR | Hazard ratio |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| IL-17RA | Interleukin-17 receptor A |
| INF | Interferon |
| IR | Incident rate |
| IRF | Interferon regulatory factor |
| LHRH | Luteinizing hormone-releasing hormone |
| MCF | Metacarpo-phalangeal |
| MCP | Monocyte chemoattractant protein |
| MHC | Major histocompatibility complex |
| MMP-3 | Metalloproteinases-3 |
| MRI | Magnetic resonance imaging |
| M-SACRAH | Modified score for the assessment and quantification of chronic rheumatoid affection of the hands |
| NO | Nitric oxide |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| O3-FAs | Omega-3 fatty acids |
| OA | Osteoarthritis |
| OMERACT-OARSI | Outcome Measures in Rheumatology Clinical Trials and Osteoarthritis Research Society International |
| OPG | Osteoprotegerin |
| PD-1 | Programmed cell death receptor |
| PDL1 | PD-1 ligand-1 |
| PG | Prostaglandin |
| PIP | Proximal inter-phalangeal |
| PR | Progesterone receptors |
| RA | Rheumatoid arthritis |
| RANKL | Receptor activator of nuclear factor-kB ligand |
| RCTs | Randomized controlled trials |
| RF | Rheumatoid factor |
| SERD | Selective estrogen receptor down-regulators |
| SERM | Selective estrogen receptor modulator |
| SjS | Sjogren syndrome |
| SLE | Systemic lupus erythematosus |
| SNPs | Single-nucleotide polymorphisms |
| SpA | Spondyloarthropaty |
| SS | Systemic sclerosis |
| SSA | Sjogren syndrome-associated autoantigen |
| TCL1A | T-cell leukemia 1A |
| TNF | Tumor necrosis factor |
| US | Ultrasound |
| VDBP | Vitamin D-binding protein |
| VDR | Vitamin D receptor |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis index |
References
- Eliyatkın, N.; Yalçın, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to a New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Hershman, D.L.; Loprinzi, C.; Schneider, B.P. Symptoms: Aromatase Inhibitor Induced Arthralgias. Adv. Exp. Med. Biol. 2015, 862, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Stearns, V. Aromatase inhibitor-associated bone and musculoskeletal effects: New evidence defining etiology and strategies for management. Breast Cancer Res. 2011, 13, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. American Society of Clinical Oncology clinical practice practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J. Clin. Oncol. 2010, 28, 3784–3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, J.; Wang, X.; Guan, Y.; Zhang, D. Aromatase inhibitors plus ovarian function suppression versus tamoxifen plus ovarian function suppression for premenopausal women with early stage breast cancer: A systematic review and meta-analysis. Ann. Palliat. Med. 2020. [Google Scholar] [CrossRef]
- Xu, X.; Chlebowski, R.T.; Shi, J.; Barac, A.; Haque, R. Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res. Treat. 2019, 174, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Belagali, Y.R.; Barkate, H.V.; Sejpal, J.J.; Parekh, B.B. Therapeutic Place of Fulvestrant in the Management of Hormone-receptor Positive Breast Cancer. Eur. Oncol. Haematol. 2016, 12, 44–50. [Google Scholar] [CrossRef] [Green Version]
- NICE National Institute for Health and Care Excellence. Managing Advanced Breast Cancer. Available online: https://pathways.nice.org.uk/pathways/advanced-breast-cancer (accessed on 28 July 2020).
- Niravath, P. Aromatase inhibitor-induced arthralgia: A review. Ann. Oncol. 2013, 24, 1443–1449. [Google Scholar] [CrossRef]
- Tenti, S.; Giordano, N.; Cutolo, M.; Giannini, F.; Fioravanti, A. Primary antiphospholipid syndrome during aromatase inhibitors therapy: A case report and review of the literature. Medicine 2019, 98, e15052. [Google Scholar] [CrossRef]
- Santen, R.J.; Brodie, H.; Simpson, E.R.; Siiteri, P.K.; Brodie, A. History of aromatase: Saga of an important biological mediator and therapeutic target. Endoc. Rev. 2009, 30, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Tomao, F.; Spinelli, G.; Vici, P.; Pisanelli, G.C.; Cascialli, G.; Frati, L.; Panici, P.B.; Tomao, S. Current role and safety profile of aromatase inhibitors in early breast cancer. Expert Rev. Anticancer Ther. 2011, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Samojlik, E.; Lipton, A.; Harvey, H.; Ruby, E.B.; Wells, S.A.; Kendall, J. Kinetic, hormonal and clinical studies with aminoglutethimide in breast cancer. Cancer 1977, 39, 2948–2958. [Google Scholar] [CrossRef]
- Kelly, C.M.; Buzdar, A.U. Anastrozole. Expert Opin. Drug Saf. 2010, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.; Chlebowski, R.; Cuzick, J.; Duffy, S.; Forbes, J.; Jonat, W.; Ravdin, P. Defining the role of aromatase inhibitors in the adjuvant endocrine treatment of early breast cancer. Curr. Med. Res. Opin. 2006, 22, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.M.; Mouret-Reynier, M.A.; Durando, X.; Van Praagh, I.; Al-Sukhun, S.; Ferriere, J.P.; Chollet, P. Comparative review of anastrozole, letrozole and exemestane in the management of early breast cancer. Expert Opin. Pharmacother. 2009, 10, 1435–1447. [Google Scholar] [CrossRef]
- Xi, J.; Ma, C.X. Sequencing Endocrine Therapy for Metastatic Breast Cancer: What Do We Do After Disease Progression on a CDK4/6 Inhibitor? Curr. Oncol. Rep. 2020, 22, 57. [Google Scholar] [CrossRef]
- Dellapasqua, S.; Colleoni, M. Letrozole. Expert Opin. Drug Metab. Toxicol. 2010, 6, 251–259. [Google Scholar] [CrossRef]
- Lintermans, A.; Neven, P.; Paridaens, R. Drug safety evaluation of exemestane. Expert Opin. Drug Saf. 2011, 10, 473–487. [Google Scholar] [CrossRef]
- Rydén, L.; Heibert Arnlind, M.; Vitols, S.; Höistad, M.; Ahlgren, J. Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo—Meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast 2016, 26, 106–114. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Cheung, K.L.; Noguchi, S.; Shao, Z.; Degboe, A.; Lichfield, J.; Thirlwell, J.; Fazal, M.; Ellis, M.J. Health-related quality of life from the FALCON phase III randomised trial of fulvestrant 500 mg versus anastrozole for hormone receptor-positive advanced breast cancer. Eur. J. Cancer 2018, 94, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lintermans, A.; Neven, P. Safety of aromatase inhibitor therapy in breast cancer. Expert Opin. Drug Saf. 2015, 14, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Stuenkel, C.A.; Davis, S.R.; Pinkerton, J.V.; Gompel, A.; Lumsden, M.A. Managing Menopausal Symptoms and Associated Clinical Issues in Breast Cancer Survivors. J. Clin. Endocrinol. Metab. 2017, 102, 3647–3661. [Google Scholar] [CrossRef] [Green Version]
- Sussman, T.A.; Kruse, M.L.; Thacker, H.L.; Abraham, J. Managing Genitourinary Syndrome of Menopause in Breast Cancer Survivors Receiving Endocrine Therapy. J. Oncol. Pract. 2019, 15, 363–370. [Google Scholar] [CrossRef] [Green Version]
- American College of Obstetrics and Gynecologists: Committee Opinion: The Use of Vaginal Estrogen in Women with a History of Estrogen-Dependent Breast Cancer. Available online: https://www.acog.org/Resources-And-Publications/Committee-Opinions/Committee-onGynecologic-Practice/The-Use-of-Vaginal-Estrogenin-Women-With-a-History-of-Estrogen-Dependent-Breast-Cancer (accessed on 28 July 2020).
- Khosrow-Khavar, F.; Filion, K.B.; Al-Qurashi, S.; Torabi, N.; Bouganim, N.; Suissa, S.; Azoulay, L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmenopausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 2017, 28, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J. Clin. Oncol. 2014, 32, 2255–2269. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Shen, G.; Liu, L.; Zhao, Q.; Lu, X.; Yang, H.; Hong, D. Aromatase inhibitors and risk of cardiovascular events in breast cancer patients: A systematic review and meta-analysis. BMC Pharmacol. Toxicol. 2019, 20, 62. [Google Scholar] [CrossRef] [Green Version]
- Pineda-Moncusí, M.; Garcia-Giralt, N.; Diez-Perez, A.; Tusquets, I.; Servitja, S.; Albanell, J.; Prieto-Alhambra, D.; Nogués, X. Thromboembolic, cardiovascular and overall mortality risks of aromatase inhibitors, compared with tamoxifen treatment: An outpatient-register-based retrospective cohort study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920909660. [Google Scholar] [CrossRef]
- Anker, G.B.; Refsum, H.; Ueland, P.M.; Johannessen, D.C.; Lien, E.A.; Lonning, P.E. Influence of aromatase inhibitors on plasma total homocysteine in postmenopausal breast cancer patients. Clin. Chem. 1999, 45, 252–256. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, C.S.; Shay, D.A.; Vieira-Potter, V.J. Cognitive Effects of Aromatase and Possible Role in Memory Disorders. Front. Endocrinol. 2018, 9, 610. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.A.; Rochon, P.A.; Moineddin, R.; Lee, P.E.; Wu, W.; Pritchard, K.I.; Tierney, M.C. Cognitive sequelae of endocrine therapy in women treated for breast cancer: A meta-analysis. Breast Cancer Res. Treat. 2018, 168, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.E.; Tierney, M.C.; Wu, W.; Pritchard, K.I.; Rochon, P.A. Endocrine treatment-associated cognitive impairment in breast cancer survivors: Evidence from published studies. Breast Cancer Res. Treat. 2016, 158, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Goss, P.E. Aromatase inhibitors and bone health in women with breast cancer. J. Clin. Oncol. 2006, 24, 5305–5312. [Google Scholar] [CrossRef] [PubMed]
- Suskin, J.; Shapiro, C.L. Osteoporosis and musculoskeletal complications related to therapy of breast cancer. Gland Surg. 2018, 7, 411–423. [Google Scholar] [CrossRef]
- Napoli, N.; Rastelli, A.; Ma, C.; Yarramaneni, J.; Vattikutti, S.; Moskowitz, G.; Giri, T.; Mueller, C.; Kulkarny, V.; Qualls, C.; et al. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER + breast cancer. Bone 2013, 55, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Oesterreich, S.; Henry, N.L.; Kidwell, K.M.; Van Poznak, C.H.; Skaar, T.C.; Dantzer, J.; Li, L.; Hangartner, T.N.; Peacock, M.; Nguyen, A.T.; et al. Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast Cancer Res. Treat. 2015, 154, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Sanz, M.; Garcia-Giralt, N.; Prieto-Alhambra, D.; Servitja, S.; Balcells, S.; Pecorelli, R.; Diez-Perez, A.; Grinberg, D.; Tusquets, I.; Nogues, X. CYP11A1 expression in bone is associated with aromatase inhibitor-related bone loss. J. Mol. Endocrinol. 2015, 55, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Goss, P.E.; Ingle, J.N.; Kubo, M.; Furukawa, Y.; Batzler, A.; Jenkins, G.D.; Carlson, E.E.; Nakamura, Y.; Schaid, D.J.; et al. Aromatase inhibitor-associated bone fractures: A case-cohort GWAS and functional genomics. Mol. Endocrinol. 2014, 28, 1740–1751. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lu, K.; Song, Y.; Zhao, S.; Ma, W.; Xuan, Q.; Tang, D.; Zhao, H.; Liu, L.; Zhang, Q. RANKL and OPG Polymorphisms Are Associated with Aromatase Inhibitor-Related Musculoskeletal Adverse Events in Chinese Han Breast Cancer Patients. PLoS ONE 2015, 10, e0133964. [Google Scholar] [CrossRef]
- Goss, P.E.; Qi, S.; Cheung, A.M.; Hu, H.; Mendes, M.; Pritzker, K.P. Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized rats. Clin. Cancer Res. 2004, 10, 5717–5723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef]
- Cheung, A.M.; Tile, L.; Cardew, S.; Pruthi, S.; Robbins, J.; Tomlinson, G.; Kapral, M.K.; Khosla, S.; Majumdar, S.; Erlandson, M.; et al. Bone density and structure in healthy postmenopausal women treated with exemestane for the primary prevention of breast cancer: A nested substudy of the MAP.3 randomised controlled trial. Lancet Oncol. 2012, 13, 275–284. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Knox, J.; Cawthorn, S.; Saunders, C.; Roche, N.; Mansel, R.E.; Von Minckwitz, G.; et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet 2014, 383, 1041–1048. [Google Scholar] [CrossRef]
- Hadji, P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit. Rev. Oncol. Hematol. 2009, 69, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Ramchand, S.K.; Cheung, Y.M.; Yeo, B.; Grossmann, M. The effects of adjuvant endocrine therapy on bone health in women with breast cancer. J. Endocrinol. 2019, 241, R111–R124. [Google Scholar] [CrossRef]
- Paschou, S.A.; Augoulea, A.; Lambrinoudaki, I. Bone health care in women with breast cancer. Hormones 2020, 19, 171–178. [Google Scholar] [CrossRef]
- Trémollieres, F.A.; Ceausu, I.; Depypere, H.; Lambrinoudaki, I.; Mueck, A.; Pérez-López, F.R.; van der Schouw, Y.T.; Senturk, L.M.; Simoncini, T.; Stevenson, J.C.; et al. Osteoporosis management in patients with breast cancer: EMAS position statement. Maturitas 2017, 95, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J. ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25, 124–137. [Google Scholar] [CrossRef]
- Grossmann, M.; Ramchand, S.K.; Milat, F.; Vincent, A.; Lim, E.; Kotowicz, M.A.; Hicks, J.; Teede, H. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: Position statement of the Endocrine Society of Australia, the Australian and New Zealand Bone & Mineral Society, the Australasian Menopause Society and the Clinical Oncology Society of Australia. Clin. Endocrinol. 2018, 89, 280–296. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Bundred, N.J.; Brufsky, A.; Coleman, R.E.; Gnant, M.; Guise, T.; Lipton, A. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: Practical guidance for prevention and treatment. Ann. Oncol. 2011, 22, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Coleman, R.; Hadji, P.; Hofbauer, L.C. Bone health during endocrine therapy for cancer. Lancet Diabetes Endocrinol. 2018, 6, 901–910. [Google Scholar] [CrossRef]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.C.; de Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J. Bone Oncol. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Pedersini, R.; Amoroso, V.; Maffezzoni, F.; Gallo, F.; Turla, A.; Monteverdi, S.; Ardine, M.; Ravanelli, M.; Vassalli, L.; Rodella, F.; et al. Association of Fat Body Mass with Vertebral Fractures in Postmenopausal Women with Early Breast Cancer Undergoing Adjuvant Aromatase Inhibitor Therapy. JAMA Netw. Open 2019, 2, e1911080. [Google Scholar] [CrossRef]
- Berruti, A.; Tucci, M.; Mosca, A.; Vana, F.; Ardine, M.; Dogliotti, L.; Angeli, A.; Bertoldo, F. Changes in bone mineral density after adjuvant aromatase inhibitors and fracture risk in breast cancer patients. J. Clin. Oncol. 2007, 25, 1455–1456. [Google Scholar] [CrossRef]
- María, R.S.; Marta, P.M.; Sonia, S.; Natalia, G.G.; Tamara, M.; Ignasi, T.; Maria, M.G.; Jaime, R.M.; Adolfo, D.P.; Joan, A.; et al. TBS and BMD at the end of AI-therapy: A prospective study of the B-ABLE cohort. Bone 2016, 92, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pineda-Moncusí, M.; Garcia-Giralt, N.; Diez-Perez, A.; Servitja, S.; Tusquets, I.; Prieto-Alhambra, D.; Nogués, X. Increased Fracture Risk in Women Treated with Aromatase Inhibitors Versus Tamoxifen: Beneficial Effect of Bisphosphonates. J. Bone Miner. Res. 2020, 35, 291–297. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Burstein, H.J. Aromatase inhibitor-associated arthralgia syndrome. Breast 2007, 16, 223–234. [Google Scholar] [CrossRef]
- Henry, N.L.; Giles, J.T.; Ang, D.; Mohan, M.; Dadabhoy, D.; Robarge, J.; Hayden, J.; Lemler, S.; Shahverdi, K.; Powers, P.; et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res. Treat. 2008, 111, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckwée, D.; Leysen, L.; Meuwis, K.; Adriaenssens, N. Prevalence of aromatase inhibitor-induced arthralgia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Rocque, G. What Is the Role of Symptom Management and Patient-Reported Outcomes in Adherence to Aromatase Inhibitors? J. Clin. Oncol. 2018, 36, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.N. Genome-wide case-control study of musculoskeletal adverse events and functional genomics in women receiving aromatase inhibitors: Going beyond associations. Breast Cancer Res. 2010, 12, S17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Giralt, N.; Rodríguez-Sanz, M.; Prieto-Alhambra, D.; Servitja, S.; Torres-del Pliego, E.; Balcells, S.; Albanell, J.; Grinberg, D.; Diez-Perez, A.; Tusquets, I.; et al. Genetic determinants of aromatase inhibitor-related arthralgia: The B-ABLE cohort study. Breast Cancer Res. Treat. 2013, 140, 385–395. [Google Scholar] [CrossRef]
- Zhu, Y.; Koleck, T.A.; Bender, C.M.; Conley, Y.P. Genetic Underpinnings of Musculoskeletal Pain during Treatment with Aromatase Inhibitors for Breast Cancer: A Biological Pathway Analysis. Biol. Res. Nurs. 2020, 22, 263–276. [Google Scholar] [CrossRef]
- Romero, S.A.; Su, H.I.; Satagopan, J.; Li, Q.S.; Seluzicki, C.M.; Dries, A.; DeMichele, A.M.; Mao, J.J. Clinical and genetic risk factors for aromatase inhibitor-associated arthralgia in breast cancer survivors. Breast 2020, 49, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Gervasini, G.; Jara, C.; Olier, C.; Romero, N.; Martínez, R.; Carrillo, J.A. Polymorphisms in ABCB1 and CYP19A1 genes affect anastrozole plasma concentrations and clinical outcomes in postmenopausal breast cancer patients. Br. J. Clin. Pharmacol. 2017, 83, 562–571. [Google Scholar] [CrossRef] [Green Version]
- Park, I.H.; Lee, Y.S.; Lee, K.S.; Kim, S.Y.; Hong, S.H.; Jeong, J.; Lee, H.; Ro, J.; Nam, B.H. Single nucleotide polymorphisms of CYP19A1 predict clinical outcomes and adverse events associated with letrozole in patients with metastatic breast cancer. Cancer Chemother. Pharmacol. 2011, 68, 1263–1271. [Google Scholar] [CrossRef]
- Fontein, D.B.; Houtsma, D.; Nortier, J.W.; Baak-Pablo, R.F.; Kranenbarg, E.M.; van der Straaten, T.R.; Putter, H.; Seynaeve, C.; Gelderblom, H.; van de Velde, C.J.; et al. Germline variants in the CYP19A1 gene are related to specific adverse events in aromatase inhibitor users: A substudy of Dutch patients in the TEAM trial. Breast Cancer Res. Treat. 2014, 144, 599–606. [Google Scholar] [CrossRef]
- Wang, J.; Lu, K.; Song, Y.; Xie, L.; Zhao, S.; Wang, Y.; Sun, W.; Liu, L.; Zhao, H.; Tang, D.; et al. Indications of clinical and genetic predictors for aromatase inhibitors related musculoskeletal adverse events in Chinese Han women with breast cancer. PLoS ONE 2013, 8, e68798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, N.L.; Skaar, T.C.; Dantzer, J.; Li, L.; Kidwell, K.; Gersch, C.; Nguyen, A.T.; Rae, J.M.; Desta, Z.; Oesterreich, S.; et al. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res. Treat. 2013, 138, 807–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niravath, P.; Chen, B.; Chapman, J.A.; Agarwal, S.K.; Welschhans, R.L.; Bongartz, T.; Kalari, K.R.; Shepherd, L.E.; Bartlett, J.; Pritchard, K.; et al. Vitamin D Levels, Vitamin D Receptor Polymorphisms, and Inflammatory Cytokines in Aromatase Inhibitor-Induced Arthralgias: An Analysis of CCTG MA.27. Clin. Breast Cancer 2018, 18, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lintermans, A.; Van Asten, K.; Jongen, L.; Van Brussel, T.; Laenen, A.; Verhaeghe, J.; Vanderschueren, D.; Lambrechts, D.; Neven, P. Genetic variant in the osteoprotegerin gene is associated with aromatase inhibitor-related musculoskeletal toxicity in breast cancer patients. Eur. J. Cancer 2016, 56, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Liang, Y.; Ou, Y.; Huang, J.; Xiong, J.; Duan, L.; Wang, D. Estrogen Modulates Cartilage and Subchondral Bone Remodeling in an Ovariectomized Rat Model of Postmenopausal Osteoarthritis. Med. Sci. Monit. 2019, 25, 3146–3153. [Google Scholar] [CrossRef]
- Tinti, L.; Niccolini, S.; Lamboglia, A.; Pascarelli, N.A.; Cervone, R.; Fioravanti, A. Raloxifene protects cultured human chondrocytes from IL-1β induced damage: A biochemical and morphological study. Eur, J. Pharmacol 2011, 670, 67–73. [Google Scholar] [CrossRef]
- Kavas, A.; Cagatay, S.T.; Banerjee, S.; Keskin, D.; Tezcaner, A. Potential of Raloxifene in reversing osteoarthritis-like alterations in rat chondrocytes: An in vitro model study. J. Biosci. 2013, 38, 135–147. [Google Scholar] [CrossRef]
- Forsblad d’Elia, H.; Mattsson, L.; Ohlsson, C.; Nordborg, E.; Carlsten, H. Hormone replacement therapy in rheumatoid arthritis is associated with lower serum levels of soluble IL-6 receptor and higher insulin-like growth factor 1. Arthritis Res. Ther. 2003, 5, R202. [Google Scholar] [CrossRef] [Green Version]
- Morales, L.; Pans, S.; Paridaens, R.; Westhovens, R.; Timmerman, D.; Verhaeghe, J.; Wildiers, H.; Leunen, K.; Amant, F.; Berteloot, P.; et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: Associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res. Treat. 2007, 104, 87–91. [Google Scholar] [CrossRef]
- Bauml, J.; Chen, L.; Chen, J.; Boyer, J.; Kalos, M.; Li, S.Q.; DeMichele, A.; Mao, J.J. Arthralgia among women taking aromatase inhibitors: Is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res. 2015, 17, 89. [Google Scholar] [CrossRef] [Green Version]
- Nahm, N.; Mee, S.; Marx, G. Efficacy of management strategies for aromatase inhibitor-induced arthralgia in breast cancer patients: A systematic review. Asia Pac. J. Clin. Oncol. 2018, 14, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2010, 119, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Alhambra, D.; Servitja, S.; Javaid, M.K.; Garrigós, L.; Arden, N.K.; Cooper, C.; Albanell, J.; Tusquets, I.; Diez-Perez, A.; Nogues, X. Vitamin D threshold to prevent aromatase inhibitor-related bone loss: The B-ABLE prospective cohort study. Breast Cancer Res. Treat. 2012, 133, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, A.L.; Taylor, M.E.; Gao, F.; Armamento-Villareal, R.; Jamalabadi-Majidi, S.; Napoli, N.; Ellis, M.J. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): A phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res. Treat. 2011, 129, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.C.; Adlis, S.A.; Robien, K.; Kirstein, M.N.; Liang, S.; Richter, S.A.; Lerner, R.E. Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res. Treat. 2016, 155, 501–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, Q.J.; Kimler, B.F.; Reddy, P.S.; Sharma, P.; Klemp, J.R.; Nydegger, J.L.; Yeh, H.W.; Fabian, C.J. Randomized trial of vitamin D3 to prevent worsening of musculoskeletal symptoms in women with breast cancer receiving adjuvant letrozole. The VITAL trial. Breast Cancer Res. Treat. 2017, 166, 491–500. [Google Scholar] [CrossRef]
- Niravath, P.; Hilsenbeck, S.G.; Wang, T.; Jiralerspong, S.; Nangia, J.; Pavlick, A.; Ademuyiwa, F.; Frith, A.; Ma, C.; Park, H.; et al. Randomized controlled trial of high-dose versus standard-dose vitamin D3 for prevention of aromatase inhibitor-induced arthralgia. Breast Cancer Res. Treat. 2019, 177, 427–435. [Google Scholar] [CrossRef]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Awad, D.; Dakhil, S.R.; Gralow, J.; Greenlee, H.; Lew, D.L.; Minasian, L.M.; Till, C.; et al. Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor-Induced Musculoskeletal Pain: SWOG S0927. J. Clin. Oncol. 2015, 33, 1910–1917. [Google Scholar] [CrossRef]
- Shen, S.; Unger, J.M.; Crew, K.D.; Till, C.; Greenlee, H.; Gralow, J.; Dakhil, S.R.; Minasian, L.M.; Wade, J.L.; Fisch, M.J.; et al. Omega-3 fatty acid use for obese breast cancer patients with aromatase inhibitor-related arthralgia (SWOG S0927). Breast Cancer Res. Treat. 2018, 172, 603–610. [Google Scholar] [CrossRef]
- Lustberg, M.B.; Orchard, T.S.; Reinbolt, R.; Andridge, R.; Pan, X.; Belury, M.; Cole, R.; Logan, A.; Layman, R.; Ramaswamy, B.; et al. Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res. Treat. 2018, 167, 709–718. [Google Scholar] [CrossRef]
- Henry, N.L.; Unger, J.M.; Schott, A.F.; Fehrenbacher, L.; Flynn, P.J.; Prow, D.M.; Sharer, C.W.; Burton, G.V.; Kuzma, C.S.; Moseley, A.; et al. Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. J. Clin. Oncol. 2018, 36, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Unger, J.M.; Till, C.; Schott, A.F.; Crew, K.D.; Lew, D.L.; Fisch, M.J.; Moinpour, C.M.; Wade, J.L., III; Hershman, D.L. Association between body mass index and response to duloxetine for aromatase inhibitor-associated musculoskeletal symptoms in SWOG S1202. Cancer 2019, 125, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, M.; Onishi, H.; Kuroki, S.; Okido, M.; Shimada, K.; Yokohata, K.; Umeda, S.; Ogawa, T.; Tanaka, M.; Katano, M. Short-term and low-dose prednisolone administration reduces aromatase inhibitor-induced arthralgia in patients with breast cancer. Anticancer Res. 2012, 32, 2331–2336. [Google Scholar]
- Greenlee, H.; Crew, K.D.; Shao, T.; Kranwinkel, G.; Kalinsky, K.; Maurer, M.; Brafman, L.; Insel, B.; Tsai, W.Y.; Hershman, D.L. Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Support. Care Cancer 2013, 21, 1077–1087. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.; Heydarian, R.; Ochoa, C.; Dwivedi, A.K.; Nahleh, Z.A. Single arm phase II study of oral vitamin B12 for the treatment of musculoskeletal symptoms associated with aromatase inhibitors in women with early stage breast cancer. Breast J. 2018, 24, 260–268. [Google Scholar] [CrossRef]
- Alhanafy, A.M.; Labeeb, A.; Khalil, A. The Role of Diuretics in Treatment of Aromatase Inhibitors Induced Musculoskeletal Symptoms in Women with Non Metastatic Breast Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 3525–3531. [Google Scholar] [CrossRef] [Green Version]
- Santa-Maria, C.A.; Bardia, A.; Blackford, A.L.; Snyder, C.; Connolly, R.M.; Fetting, J.H.; Hayes, D.F.; Jeter, S.C.; Miller, R.S.; Nguyen, A.; et al. A phase II study evaluating the efficacy of zoledronic acid in prevention of aromatase inhibitor-associated musculoskeletal symptoms: The ZAP trial. Breast Cancer Res. Treat. 2018, 171, 121–129. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Dudler, J.; Blicharski, T.; Pavelka, K. Pharmaceutical-grade Chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: The ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT). Ann. Rheum. Dis. 2017, 76, 1537–1543. [Google Scholar] [CrossRef] [Green Version]
- Tenti, S.; Giordano, N.; Mondanelli, N.; Giannotti, S.; Maheu, E.; Fioravanti, A. A retrospective observational study of glucosamine sulfate in addition to conventional therapy in hand osteoarthritis patients compared to conventional treatment alone. Aging Clin. Exp. Res. 2020, 32, 1161–1172. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.M.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Xepapadakis, G.; Ntasiou, P.; Koronarchis, D.; Koufoudakis, D.; Panousis, D.; Grosomanidis, D.; Venizelos, V.; Georgiadis, S. New views on treatment of aromatase inhibitors induced arthralgia. Breast 2010, 19, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Briot, K.; Tubiana-Hulin, M.; Bastit, L.; Kloos, I.; Roux, C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: The ATOLL (articular tolerance of letrozole) study. Breast Cancer Res. Treat. 2010, 120, 127–134. [Google Scholar] [CrossRef] [PubMed]
- COSA Exercise and Cancer Group Executive Committee. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med. J. Aust. 2019, 210, 54–54.e1. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Lohrisch, C.A.; McKenzie, D.; Truong, P.; Jesperson, D.; Gelmon, K.A.; Premji, S.; Kennecke, H.F. A randomized trial of exercise versus control for musculoskeletal symptoms from adjuvant anastrozole (A) for postmenopausal early breast cancer (PEBC). J. Clin. Oncol. 2011, 29, 636. [Google Scholar] [CrossRef]
- Irwin, M.L.; Cartmel, B.; Gross, C.P.; Ercolano, E.; Li, F.; Yao, X.; Fiellin, M.; Capozza, S.; Rothbard, M.; Zhou, Y.; et al. Randomized exercise trial of aromatase inhibitor–induced arthralgia in breast cancer survivors. J. Clin. Oncol. 2015, 33, 1104–1111. [Google Scholar] [CrossRef]
- Fields, J.; Richardson, A.; Hopkinson, J.; Fenlon, D. Nordic walking as an exercise intervention to reduce pain in women with aromatase inhibitor- associated arthralgia: A feasibility study. J. Pain Symptom Manag. 2016, 52, 548–559. [Google Scholar] [CrossRef] [Green Version]
- Varadarajan, R.; Helm, E.; Arnold, C.; Huelsenbeck-Dill, L.; Ingraham-Lopresto, B.; Sonaad, S.; Swanson, P.; Sims-Mourtada, J.; Dickson-Whitmer, D. Abstract P5-12-04: Directed exercise intervention in breast cancer patients with arthralgias receiving aromatase inhibitors: A randomized pilot study. Cancer Res. 2016, 76, P5–P12. [Google Scholar]
- Nyrop, K.; Callahan, L.; Cleveland, R.; Arbeeva, L.; Hackney, B.; Muss, H. Randomized controlled trial of a home-based walking program to reduce moderate to severe aromatase inhibitor associated arthralgia in breast cancer survivors. Oncologist 2017, 22, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Sanmugarajah, J.; Allan, S.; Bagchi, R.; Laakso, E.L. Can a supervised exercise program compared to usual care prevent aromatase inhibitor-induced musculoskeletal pain in women with breast cancer? Cancer Res. 2017, 77, P5–P12. [Google Scholar]
- Tajaesu, M.; Tamaki, K.; Nagamine, S.; Kamada, Y.; Uehara, K.; Arakaki, M.; Tamatsu, Y.; Yamashiro, K.; Miyashita, M.; Ishida, T.; et al. Final results of the randomized trial of exercise intervention vs. usual care for breast cancer patients with aromatase inhibitor to prevent and improve the aromatase inhibitor induced arthralgia. Cancer Res. 2018, 78, P6–P11. [Google Scholar]
- Lu, G.; Zheng, J.; Zhang, L. The effect of exercise on aromatase inhibitor-induced musculoskeletal symptoms in breast cancer survivors: A systematic review and meta-analysis. Support. Care Cancer 2020, 28, 1587–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, K.E.; Rickett, K.; Feng, S.; Vagenas, D.; Woodward, N.E. Exercise therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer. Cochrane Database Syst. Rev. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Niravath, P. Acupuncture and Vitamin D for the Management of Aromatase Inhibitor-Induced Arthralgia. Curr. Oncol. Rep. 2019, 21, 51. [Google Scholar] [CrossRef]
- Crew, K.D.; Capodice, J.L.; Greenlee, H.; Brafman, L.; Fuentes, D.; Awad, D.; Yann Tsai, W.; Hershman, D.L. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early-stage breast cancer. J. Clin. Oncol. 2010, 28, 1154–1160. [Google Scholar] [CrossRef]
- Hershman, D.L.; Unger, J.M.; Greenlee, H.; Capodice, J.L.; Lew, D.L.; Darke, A.K.; Kengla, A.T.; Melnik, M.K.; Jorgensen, C.W.; Kreisle, W.H.; et al. Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA 2018, 320, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Bao, T.; Cai, L.; Giles, J.T.; Gould, J.; Tarpinian, K.; Betts, K.; Medeiros, M.; Jeter, S.; Tait, N.; Chumsri, S.; et al. A dual-center randomized controlled double blind trial assessing the effect of acupuncture in reducing musculoskeletal symptoms in breast cancer patients taking aromatase inhibitors. Breast Cancer Res. Treat. 2013, 138, 167–174. [Google Scholar] [CrossRef]
- Oh, B.; Kimble, B.; Costa, D.S.; Davis, E.; McLean, A.; Orme, K.; Beith, J. Acupuncture for treatment of arthralgia secondary to aromatase inhibitor therapy in women with early breast cancer: Pilot study. Acupunct. Med. 2013, 31, 264–271. [Google Scholar] [CrossRef]
- Mao, J.J.; Xie, S.X.; Farrar, J.T.; Stricker, C.T.; Bowman, M.A.; Bruner, D.; DeMichele, A. A randomised trial of electro-acupuncture for arthralgia related to aromatase inhibitor use. Eur. J. Cancer 2014, 50, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Morel, B.; Marotte, H.; Miossec, P. Will steroidal aromatase inhibitors induce rheumatoid arthritis? Ann. Rheum. Dis. 2007, 66, 557–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzzese, V.; Hassan, C.; Zullo, A.; Zampa, G. Rheumatoid arthritis: A complication of aromatase inhibitor therapy? Int. J. Immunopathol. Pharmacol. 2011, 24, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, E.; Letho-Gyselinck, H.; Prati, C.; Wendling, D. Rheumathoid arthritis and aromatase inhibitors. Jt. Bone Spine 2011, 78, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Parker, B.A.; Zvaifler, N.J. Accelerated cutaneous nodulosis associated with aromatase inhibitor therapy in a patient with rheumatoid arthritis. J. Rheumatol. 2009, 36, 1087–1088. [Google Scholar] [CrossRef]
- Scarpa, R.; Atteno, M.; Peluso, R.; Costa, L.; Padula, S.; Di Minno, D.; Caso, F.; Iervolino, S.; Vitiello, M.; Del Puente, A. Rheumatic complaints in women taking aromatase inhibitors for treatment of hormone-dependent breast cancer. J. Clin. Rheumatol. 2011, 17, 169–172. [Google Scholar] [CrossRef]
- Laroche, M.; Borg, S.; Lassoued, S.; De Lafontan, B.; Roché, H. Joint pain with aromatase inhibitors: Abnormal frequency of Sjögren’s syndrome. J. Rheumatol. 2007, 34, 2259–2263. [Google Scholar]
- Guidelli, G.M.; Martellucci, I.; Galeazzi, M.; Francini, G.; Fioravanti, A. Sjögren’s syndrome and aromatase inhibitors treatment: Is there a link? Clin. Exp. Rheumatol. 2013, 31, 653–654. [Google Scholar]
- Yasar Bilge, N.S.; Korkmaz, C. Does Aromatase Inhibitors Cause Sjogren’s Syndrome and Polyneuropathy? World J. Oncol. 2014, 5, 181–182. [Google Scholar] [CrossRef]
- Pokhai, G.; Buzzola, R.; Abrudescu, A. Letrozole-induced very early systemic sclerosis in a patient with breast cancer: A case report. Arch. Rheumatol. 2014, 29, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Mascella, F.; Gianni, L.; Affatato, A.; Fantini, M. Aromatase inhibitors and antisynthetase syndrome. Int. J. Immunopathol. Pharmacol. 2016, 29, 494–497. [Google Scholar] [CrossRef] [Green Version]
- Creamer, P.; Lim, K.; George, E.; Dieppe, P. Acute inflammatory polyarthritis in association with tamoxifen. Br. J. Rheumatol. 1994, 33, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.J. Acute inflammatory polyarthritis in association with tamoxifen. Br. J. Rheumatol. 1994, 33, 998. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Howell, F.V.; Bone, R.C.; Michelson, P. Primary Sjogren syndrome: Clinical and immunopathologic features. Semin. Arthritis Rheum. 1984, 14, 77–105. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. European Study Group on Classification Criteria for Sjögren’s SyndromeClassification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.H.; Derksen, R.H.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Sherer, Y.; Kuechler, S.; Jose Scali, J.; Rovensky, J.; Levy, Y.; Zandman-Goddard, G.; Shoenfeld, Y. Low dose intravenous immunoglobulin in systemic lupus erythematosus: Analysis of 62 cases. Isr. Med. Assoc. J. 2008, 10, 55–57. [Google Scholar]
- Tenti, S.; Guidelli, G.M.; Bellisai, F.; Galeazzi, M.; Fioravanti, A. Long-term treatment of antiphospholipid syndrome with intravenous immunoglobulin in addition to conventional therapy. Clin. Exp. Rheumatol. 2013, 31, 877–882. [Google Scholar]
- Tenti, S.; Fabbroni, M.; Mancini, V.; Russo, F.; Galeazzi, M.; Fioravanti, A. Intravenous Immunoglobulins as a new opportunity to treat discoid lupus erythematosus: A case report and review of the literature. Autoimmun. Rev. 2018, 17, 791–795. [Google Scholar] [CrossRef]
- Inno, A.; Basso, M.; Vecchio, F.M.; Marsico, V.A.; Cerchiaro, E.; D’Argento, E.; Bagalà, C.; Barone, C. Anastrozole-related acute hepatitiswith autoimmune features: A case report. BMC Gastroenterol. 2011, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Wright, G.; Tanner, P.; Lucas, R. A case of anastrazole-related drug-induced autoimmune hepatitis. Clin. J. Gastroenterol. 2014, 7, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Klapko, O.; Ghoulam, E.; Jakate, S.; Eswaran, S.; Usha, L. Anastrozole-induced autoimmune hepatitis: A rare complication of breast cancer therapy. Anticancer Res. 2017, 37, 4173–4176. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Cohen, P.R. Anastrozole-Induced Dermatitis: Report of a Woman with an Anastrozole-Associated Dermatosis and a Review of Aromatase Inhibitor-Related Cutaneous Adverse Events. Dermatol. Ther. 2020, 10, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarhan, F.; Keser, G.; Alacacıoğlu, A.; Akar, S. Rheumatological Findings in Patients with Breast Cancer. Eur. J. Breast Health 2019, 16, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ballou, S.P. The effect of antiestrogen agents on risk of autoimmune disorders in patients with breast cancer. J. Rheumatol. 2015, 42, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, M.; Carrara, G.; Sakellariou, G.; Silvagni, E.; Scirè, C.A. Influence of aromatase inhibitors therapy on the occurrence of rheumatoid arthritis in women with breast cancer: Results from a large population-based study of the Italian Society for Rheumatology. RMD Open 2017, 3, e000523. [Google Scholar] [CrossRef]
- Chien, H.C.; Kao Yang, Y.H.; Kwoh, C.K.; Chalasani, P.; Wilson, D.L.; Lo-Ciganic, W.H. Aromatase Inhibitors and Risk of Arthritis and Carpal Tunnel Syndrome among Taiwanese Women with Breast Cancer: A Nationwide Claims Data Analysis. J. Clin. Med. 2020, 9, 566. [Google Scholar] [CrossRef] [Green Version]
- Wadström, H.; Pettersson, A.; Smedby, K.E.; Askling, J. Risk of breast cancer before and after rheumatoid arthritis, and the impact of hormonal factors. Ann. Rheum. Dis. 2020, 79, 581–586. [Google Scholar] [CrossRef]
- Rossi, E.; Morabito, A.; Di Rella, F.; Esposito, G.; Gravina, A.; Labonia, V.; Landi, G.; Nuzzo, F.; Pacilio, C.; De Maio, E.; et al. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: The HOBOE trial. J. Clin. Oncol. 2009, 27, 3192–3197. [Google Scholar] [CrossRef]
- Ingle, J.N.; Buzdar, A.U.; Schaid, D.J.; Goetz, M.P.; Batzler, A.; Robson, M.E.; Northfelt, D.W.; Olson, J.E.; Perez, E.A.; Desta, Z.; et al. Variation in anastrozole metabolism and pharmacodynamics in women with early breast cancer. Cancer Res. 2010, 70, 3278–3286. [Google Scholar] [CrossRef] [Green Version]
- Gallicchio, L.; Macdonald, R.; Wood, B.; Rushovich, E.; Helzlsouer, K.J. Androgens and musculoskeletal symptoms among breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res. Treat. 2011, 130, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Straub, R.H. Estrogen metabolism and autoimmunity. Autoimmun. Rev. 2012, 11, A460–A464. [Google Scholar] [CrossRef] [PubMed]
- Capellino, S.; Straub, R.H.; Cutolo, M. Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: Common pathway in both sexes. Ann. N. Y. Acad. Sci. 2014, 1317, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Merrheim, J.; Villegas, J.; Van Wassenhove, J.; Khansa, R.; Berrih-Aknin, S.; le Panse, R.; Dragin, N. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmun. Rev. 2020, 19, 102468. [Google Scholar] [CrossRef] [PubMed]
- Jingxuan, W.; Qingyuan, Z.; Shi, J.; Meiyan, F.; Xinmei, K.; Shu, Z.; Shuling, L.; Wenhui, Z. Immoderate inhibition of estrogen by anastrozole enhances the severity of experimental polyarthritis. Exp. Gerontol. 2009, 44, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Generali, D.; Bates, G.; Berruti, A.; Brizzi, M.P.; Campo, L.; Bonardi, S.; Bersiga, A.; Allevi, G.; Milani, M.; Aguggini, S.; et al. Immunomodulation of FOXP3+ regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin. Cancer Res. 2009, 15, 1046–1051. [Google Scholar] [CrossRef] [Green Version]
- Correale, P.; Saladino, R.E.; Nardone, V.; Giannicola, R.; Agostino, R.; Pirtoli, L.; Caraglia, M.; Botta, C.; Tagliaferri, P. Could PD-1/PDL1 immune checkpoints be linked to HLA signature? Immunotherapy 2019, 11, 1523–1526. [Google Scholar] [CrossRef]
- Shim, G.J.; Warner, M.; Kim, H.J.; Andersson, S.; Liu, L.; Ekman, J.; Imamov, O.; Jones, M.E.; Simpson, E.R.; Gustafsson, J.Å. Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren’s syndrome. Proc. Natl. Acad. Sci. USA 2004, 101, 12628–12633. [Google Scholar] [CrossRef] [Green Version]
- Medina, K.L.; Strasser, A.; Kincade, P.W. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood 2000, 95, 2059–2067. [Google Scholar] [CrossRef]
- Iwasa, A.; Arakaki, R.; Honma, N.; Ushio, A.; Yamada, A.; Kondo, T.; Kurosawa, E.; Kujiraoka, S.; Tsunematsu, T.; Kudo, Y.; et al. Aromatase controls Sjögren syndrome-like lesions through monocyte chemotactic protein-1 in target organ and adipose tissue-associated macrophages. Am. J. Pathol. 2015, 185, 151–161. [Google Scholar] [CrossRef]
- Maurizi, G.; Poloni, A.; Mattiucci, D.; Santi, S.; Maurizi, A.; Izzi, V.; Giuliani, A.; Mancini, S.; Zingaretti, M.C.; Perugini, J.; et al. Human White Adipocytes Convert into “Rainbow” Adipocytes In Vitro. J. Cell. Physiol. 2017, 232, 2887–2899. [Google Scholar] [CrossRef]
- Van Raemdonck, K.; Umar, S.; Szekanecz, Z.; Zomorrodi, R.K.; Shahrara, S. Impact of obesity on autoimmune arthritis and its cardiovascular complications. Autoimmun. Rev. 2018, 17, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Tenti, S.; Bacarelli, M.R.; Damiani, A.; Li Gobbi, F.; Bandinelli, F.; Cheleschi, S.; Galeazzi, M.; Benucci, M. Tocilizumab modulates serum levels of adiponectin and chemerin in patients with rheumatoid arthritis: Potential cardiovascular protective role of IL-6 inhibition. Clin. Exp. Rheumatol. 2019, 37, 293–300. [Google Scholar] [PubMed]
- Nguyen, M.C.; Stewart, R.B.; Banerji, M.A.; Gordon, D.H.; Kral, J.G. Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int. J. Obes. 2001, 25, 296–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Londen, G.J.; Perera, S.; Vujevich, K.; Rastogi, P.; Lembersky, B.; Brufsky, A.; Vogel, V.; Greenspan, S.L. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res. Treat. 2011, 125, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibb, F.W.; Dixon, J.M.; Clarke, C.; Homer, N.Z.; Faqehi, A.M.; Andrew, R.; Walker, B.R. Higher Insulin Resistance and Adiposity in Postmenopausal Women with Breast Cancer Treated with Aromatase Inhibitors. J. Clin. Endocrinol. Metab. 2019, 104, 3670–3678. [Google Scholar] [CrossRef]
- Melillo, N.; Cantatore, F.P. Breast cancer anti-hormonal therapy and rheumatic diseases: Linking the clinical to molecular world. Beyond Rheumatol. 2020, 2, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, L.; Bongartz, T.; Hawse, J.R.; Markovic, S.N.; Schaid, D.J.; Mushiroda, T.; Kubo, M.; Nakamura, Y.; Kamatani, N.; et al. Aromatase inhibitors, estrogens and musculoskeletal pain: Estrogen-dependent T-cell leukemia 1A (TCL1A) gene-mediated regulation of cytokine expression. Breast Cancer Res. 2012, 14, R41. [Google Scholar] [CrossRef] [Green Version]
- Araujo, E.G.; Schett, G. Enthesitis in psoriatic arthritis (Part 1): Pathophysiology. Rheumatology 2020, 59, i10–i14. [Google Scholar] [CrossRef] [Green Version]
- Kramer, P.R.; Winger, V.; Kramer, S.F. 17beta-Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes. Mol. Cell. Endocrinol. 2007, 279, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Henry, N.L.; Pchejetski, D.; A’hern, R.; Nguyen, A.T.; Charles, P.; Waxman, J.; Li, L.; Storniolo, A.M.; Hayes, D.F.; Flockhart, D.A.; et al. Inflammatory cytokines and aromatase inhibitor-associated musculoskeletal syndrome: A case-control study. Br. J. Cancer 2010, 103, 291–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, M.; Corinaldesi, C.; Antinozzi, C.; Crescioli, C. Vitamin D in autoimmune rheumatic diseases: A view inside gender differences. Pharmacol. Res. 2017, 117, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and Autoimmune Diseases: Is Vitamin D Receptor (VDR) Polymorphism the Culprit? Isr. Med. Assoc. J. 2017, 19, 438–443. [Google Scholar] [PubMed]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Vitamin D and autoimmune diseases. Life Sci. 2019, 233, 116744. [Google Scholar] [CrossRef]
- Garbossa, S.G.; Folli, F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev. Endocr. Metab. Disord. 2017, 18, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Villaggio, B.; Soldano, S.; Cutolo, M. 1, 25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin. Exp. Rheumatol. 2012, 30, 934–938. [Google Scholar]
- Imtiaz, S.; Siddiqui, N.; Raza, S.A.; Loya, A.; Muhammad, A. Vitamin D deficiency in newly diagnosed breast cancer patients. Indian J. Endocrinol. Metab. 2012, 16, 409–413. [Google Scholar] [CrossRef]
- Coleman, R.E.; Rathbone, E.J.; Marshall, H.C.; Wilson, C.; Brown, J.E.; Gossiel, F.; Gregory, W.M.; Cameron, D.; Bell, R. Vitamin D, but not bone turnover markers, predict relapse in women with early breast cancer: An AZURE translational study. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Espinosa, A.; Dardalhon, V.; Brauner, S.; Ambrosi, A.; Higgs, R.; Quintana, F.J.; Sjöstrand, M.; Eloranta, M.L.; Ní Gabhann, J.; Winqvist, O.; et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J. Exp. Med. 2009, 206, 1661–1671. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Zhong, C.; Hu, J.; Hu, H.; Zhou, D.; Cao, M. Decreased expression of TRIM21 indicates unfavorable outcome and promotes cell growth in breast cancer. Cancer Manag. Res. 2018, 10, 3687–3696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, S.C.; Simmonds, M.J. The HLA region and autoimmune disease: Associations and mechanisms of action. Curr. Genomics 2007, 8, 453–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, A.G.; Horgan, P.G.; Edwards, J. The bodies fight against cancer: Is human leucocyte antigen (HLA) class 1 the key? J. Cancer Res. Clin. Oncol. 2012, 138, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Licchetta, A.; Correale, P.; Migali, C.; Remondo, C.; Francini, E.; Pascucci, A.; Magliocca, A.; Guarnieri, A.; Savelli, V.; Piccolomini, A.; et al. Oral metronomic chemo-hormonal-therapy of metastatic breast cancer with cyclophosphamide and megestrol acetate. J. Chemother. 2010, 22, 201–204. [Google Scholar] [CrossRef]
- Scott, D.L. Biologics-based therapy for the treatment of rheumatoid arthritis. Clin. Pharmacol. Ther. 2012, 91, 30–43. [Google Scholar] [CrossRef]
- Seror, R.; Mariette, X. Malignancy and the Risks of Biologic Therapies: Current Status. Rheum. Dis. Clin. N. Am. 2017, 43, 43–64. [Google Scholar] [CrossRef]
- Rocca, A.; Maltoni, R.; Bravaccini, S.; Donati, C.; Andreis, D. Clinical utility of fulvestrant in the treatment of breast cancer: A report on the emerging clinical evidence. Cancer Manag. Res. 2018, 10, 3083–3099. [Google Scholar] [CrossRef] [Green Version]
- Di Leo, A.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 2010, 28, 4594–4600. [Google Scholar] [CrossRef]
- Gossec, L.; Kedra, J.; Servy, H.; Pandit, A.; Stones, S.; Berenbaum, F.; Finckh, A.; Baraliakos, X.; Stamm, T.A.; Gomez-Cabrero, D.; et al. EULAR points to consider for the use of big data in rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2020, 79, 69–76. [Google Scholar] [CrossRef]

| Major criteria |
| Currently taking AIs therapy |
| Joint pain which has developed or worsened since starting AIs therapy |
| Joint pain improves or resolves within 2 weeks of stopping AIs therapy |
| Joint pain returns upon resuming AIs |
| Minor criteria |
| Symmetrical joint pains |
| Pain in hands and/or wrists |
| Carpal tunnel syndrome |
| Decreased grip strength |
| Morning stiffness |
| Improvement in joint discomfort with use or exercise |
| Authors | Study Design | Pts (no) | AIs | Interval Time between AIs Starting and the Studied Treatment | Interventions Arms | Study Duration | Follow-Up Duration | Adherence to the Whole Protocol | Significant Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Khan et al. [84] 2010 | Prospective study | 60 | LTZ | 4 weeks | Arm 1 (47 women with 25OHD levels ≤40 ng/mL): 50,000 IU of oral VitD3/week Arm 2 (13 women with a 25OHD level > 40 ng/mL): calcium 1200 mg/day and VitD3 600 IU/day | 12 weeks | 12 weeks | 85% | Higher (p = 0.059) improvement of HAQ-II in arm 1 vs arm 2 at the end of the therapy. No significant change of BFI, MEN-QOL and subjective joint pain between the two groups |
| Prieto-Alhambra et al. [85] 2011 | Prospective not controlled study | 260 | N.R. | Started together | Arm 1: oral 16,000 IU VitD3 every 2 weeks, in addition to oral calcium (1 g) and VitD3 (800 IU) daily | 3 months | 3 months | 97.6% | VAS joint pain was significantly (p = 0.02) attenuated in patients reaching concentrations of 25OHD of ≥40 ng/mL, with a lower risk of incident arthralgia |
| Rastelli et al. [86] 2011 | RCT | 60 | ANA | 8 weeks | Stratum A (women with 25OHD levels 20–29 ng/mL): oral 50,000 IU VitD2 (Arm 1) or oral placebo (Arm 2) weekly for 8 weeks, then monthly Stratum B (women with 25OHD levels 10–19 ng/mL): oral 50,000 IU VitD2 weekly (Arm 1) or oral placebo (Arm 2) for 16 weeks and then monthly | 6 months | 6 months | 78% | Pain severity, as measured by FIQ and BPI-SF significantly decreased in patients treated with VitD vs placebo after 2 months, but at 6 months follow-up there were no significant differences |
| Shapiro et al. [87] 2016 | RCT | 116 | LTZ: 55 pts ANA: 47 pts EXE: 11 pts | Mean ± SD: 19.9 ± 17 months | Arm 1 (56): oral 600 IU VitD3 plus 1000 mg calcium carbonate daily Arm 2 (57): oral 4000 IU VitD3 plus 1000 mg calcium carbonate daily | 6 months | 6 months | 95% | No significant differences between the groups in BCPT-MS scale, PROMIS score, HGST, AUSCAN and WOMAC at 6 months |
| Khan et al. [88] 2017 | RCT | 160 | LTZ | Started together | Arm 1 (80 pts): oral 30,000 IU VitD3 weekly, in addition to 1200 mg of calcium and 600 IU of VitD3 daily Arm 2 (80 pts): oral placebo weekly, in addition to 1200 mg of calcium and 600 IU of VitD3 daily | 24 weeks | 24 weeks | 91% | 30,000 IU VitD3 weekly failed to show a benefit in preventing new or worsening AIA based on the protocol defined primary endpoints (HAQ-II, CPIS, LTZ discontinuation) |
| Niravath et al. [89] 2019 | RCT | 93 | N.R. | Started together | Arm 1 (46 pts): oral 50,000 IU VitD3 weekly for 12 weeks, followed by 2,000 IU daily for 40 weeks Arm 2 (47 pts): oral 800 IU VitD3 daily for 52 weeks | 52 weeks | 52 weeks | 89% | 12 weeks after randomization, 57% from arm 2 and 54% from arm 1 developed AIA (defined as an increase of HAQ-II ≥ 0.2 and/or an increase of VAS pain ≥ 0.3) and the study was terminated early for futility |
| Hershman et al. [90] 2015 | RCT | 249 | ANA: 146 pts EXE: 29 pts LTZ: 74 pts | Median: 1.2 years | Arm 1 (122 pts): oral O3-FAs 3.3 g daily Arm 2 (127 pts): matching placebo | 24 weeks | 24 weeks | 99% | No differences between the groups both at 12 and 24 weeks in the primary (BPI) and secondary (M-SACRAH, WOMAC and FACT-ES) endpoints |
| Shen et al. [91] 2018 | Exploratory analysis of the study by Hershman [88] in obese pts | 110 | ANA: 60 pts EXE: 13 pts LTZ: 37 pts | Median: 1.33 years | Arm 1: oral O3-FAs 3.3 g daily Arm 2: matching placebo daily | 24 weeks | 24 weeks | N.R. | O3-FAs therapy was associated with significant lower BPI scores at 24 weeks vs placebo. Furthermore, a statistically significant improvement in Global Ratings of Change scores for joint pain and stiffness and of M-SACRAH and WOMAC was observed in Arm 1 vs. placebo |
| Lutsberg et al. [92] 2018 | RCT | 44 | ANA: 31 pts EXE: 1 pt LTZ: 12 pts | Less than 21 days | Arm 1 (22 pts): oral 4.3 g/day of n–3 PUFAs Arm 2 (22 pts): matching placebo | 24 weeks | 24 weeks | 86% | Pain severity scores measured by BPI-SF didn’t change significantly by time or treatment arm. A significant difference in quality of life, based on FACT-ES scores, was observed in arm 1 vs. placebo in the short-term (12 weeks) |
| Henry et al. [93] 2018 | RCT | 289 | N.R. | At least 21 days Mean: 47.9 ± 36.3 weeks | Arm 1 (145 pts): oral Duloxetine 30 mg daily for 1 week, followed by 60 mg daily for 11 weeks, followed by 30 mg daily for another week Arm 2 (144 pts): matching placebo | 13 weeks | 24 weeks | 75% | A greater significant reduction of average joint pain (by BPI-SF) was reported in Arm 1 vs placebo at 12 weeks, but not at 24 weeks. Furthermore, a significant improvement of WOMAC, M-SACRAH and FACT-ES was observed in the Duloxetine arm |
| Henry et al. [94] 2019 | Exploratory analysis of the study by Henry et al. [95] on the basis of BMI categories | 289 | N.R. | Mean: 47.9 ± 36.3 weeks | Arm 1 (145 pts, of whose 78 obese): oral Duloxetine 30 mg daily for 1 week, followed by 60 mg daily for 11 weeks, followed by 30 mg daily for another week Arm 2 (144 pts, of whose 78 obese): matching placebo | 13 weeks | 24 weeks | 75% | The reduction of pain measured by BPI-SF, was more pronounced in obese patients treated with Duloxetine vs placebo at 12 weeks, while it was similar to placebo in the non-obese group. Similar findings were reported for M-SACRAH, WOMAC, FACT-ES |
| Kubo et al. [96] 2012 | Prospective not controlled study | 27 | ANA:25 pts LTZ: 2 pts | Mean: 16 months | Arm 1: 5 mg of oral Prednisolone once a day for one week | 1 week | 2 months | 100% | Joint pain symptoms, measured by VAS, improved in 67% of pts immediately after Prednisolone use, with persistent effect at one month in 63% and at 2 months in 52% |
| Greenlee et al. [97] 2013 | Prospective not controlled study | 53 | ANA: 35 pts EXE: 3 pts LTZ:2 pts | At least 3 months | Arm 1: 2 capsulesx3 times/day or 3 capsulesx2 times/day, each capsule containing 250 mg Glucosamine sulfate potassium chloride and 200 mg Chondroitin sulfate sodium | 24 weeks | 24 weeks | 69.8% | At week 24, 46.2% of pts met the OMERACT-OARSI criteria for self-reported improvements in pain and function, as measured by BPI, WOMAC and M-SACRAH |
| Campbell et al. [98] 2017 | Prospective not controlled study | 41 | N.R. | At least 14 days | Arm 1: 2500 mcg of sublingual vitB12 daily | 3 months | 3 months | 87.8% | After 3 months, a 23% relative improvement from baseline in worst pain score (by BPI-SF) and 34% in average pain score (BPI-SF) was found. Also, FACT-ES score significantly improved |
| Alhanafy et al. [99] 2018 | Prospective not controlled study | 50 | N.R. | <1 year: 12 pts 1–3 years: 29 pts >3 years: 9 pts | Arm 1: oral combination of Frusemide 20 mg/Spironolactone 50 mg once a day | 4 weeks | 4 weeks | 92% | All WOMAC sub-scores and quick DASH score significantly improved at the end of the treatment vs. baseline |
| Santa-Maria et al. [100] 2018 | Prospective not controlled study | 59 | LTZ | Letrozole was started 1–2 weeks following the initial dose of zolendronic acid | Arm 1: 4 mg of i.v. zolendronic acid at baseline and at 6 months | 6 months | 12 months | 88% | A significantly lower incidence of AIA (defined as an increase of 0.22 in HAQ-II and/or an increase of 2 cm in a VAS 0–10) after 1 year was shown in patients receiving zoledronic acid, compared with historical controls from the ELPh trial |
| Authors | Study Design | Pts (no) | AIs | Time from AIs Therapy and Symptoms Onset | Time from AIs Therapy and Diagnosis | Diagnosis | Autoimmune Laboratory Findings | Treatment for the Rheumatic Disease | Improvement after AIs Discontinuation |
|---|---|---|---|---|---|---|---|---|---|
| Morel et al. [123] 2007 | Case report | 1 | EXE for 4 months | few days | 4 months | RA | RF -; anti-CPP - | MTX 15 mg/week | No |
| Bruzzese et al. [124] 2011 | Case report | 1 | ANA for 4 years | 1 year | 5 years | RA | RF +; anti-CCP +; Antinuclear ab -; ENA - | MTX 15 mg/week, Methylprednisolone 16 mg/day | No |
| Bertolini et al. [125] 2011 | Case series | 3 | LTZ for 3 months, followed by EXE for 2 months (1 pt); ANA for 6 months (1 pt); LTZ for 4 months, followed by EXE for one month (1 pt) | Two weeks (1 pt); few weeks (1 pt); 4 months (1 pt) | One year (1 pt); 4 years (1 pt); 3 years (1 pt) | RA (3 pts) | Anti-CCP + (3 pts); RF + (2 pts); Antinuclear ab + 1/160 (2 pts); Antinuclear ab + 1/640 (1 pt) | HCQ 200 mg × 2 times/day (1 pt); SSZ 2 g/day (1 pt); Prednisone 10 mg/day (1 pt) | No (3 pts) |
| Chao et al. [126] 2009 | Case report | 1 | LTZ for 16 months | 16 months | 16 months | Accelerated cutaneous nodulosis in pt with RA history | RF+; anti-CCP + | None | Yes (the nodules decreased in size and tenderness) |
| Scarpa et al. [127] 2011 | Descriptive cross-sectional study | 18 | Type of AIs N.R. Mean duration of the therapy: 12 months | N.R. | N.R. | Undifferentiated SpA (10 pts); oligoarthritis (2 pts); arthralgia (6 pts) | Anti-CCP + (1 pt); RF − (18 pts) | NSAIDs (11 pts), corticosteroids (5 pts), MTX 10 mg/week (3 pts) | Yes (2 pts). N.R. (16 pts) |
| Laroche et al. [128] 2007 | Observational study | 24 | ANA (20 pts) and LTZ (4 pts); Duration of the therapy: N.R. | 2.5 months (mean time) | N.R. | Probable SjS (7 pts); definite SjS (1 pt); RA (1 pt); Hashimoto thyroiditis (1 pt); HCV (2 pts); shoulder tendinitis (1 pt); paraneoplastic aponeurositis (1 pt); OA (2 pts); unknown (7 pts) | Antinuclear ab + >1/160 (9 pts); RF + (4 pts); anti-CCP (2 pts) | NSAIDs (19 pts), Prednisone 10 mg/day for 8 days (9 pts) | N.R. |
| Guidelli et al. [129] 2012 | Case series | 3 | ANA for 2 years (1 pt); ANA for 3 years (1 pt); LTZ for 3 years (1 pt) | 3 months (2 pts); 5 months (1 pt) | 1 year (3 pts) | SjS | RF + (2 pts); Antinuclear ab+ 1/320 (2 pts): anti-Ro-SSA + (2 pts); anti-CCP - (3 pts) | N.R. | N.R. |
| Yasar Bilge et al. [130] 2014 | Case report | 1 | ANA Duration of the therapy: N.R. | N.R. | 3 years | SjS and polyneuropathy | RF +; Antinuclear ab+; anti-SSA and SSB - | IVIG treatment (400 mg/kg/day for 5 days monthly for 6 months) | N.R. |
| Pokhai et al. [131] 2014 | Case report | 1 | LTZ for 4 years, then EXE | 2 years | 4 years | SS | Antinuclear ab+ 1/1280 with centromeric pattern; anti-centromere B + | N.R. | Yes (an improvement was noted after LTZ discontinuation and substitution with EXE |
| Mascella et al. [132] 2016 | Case report | 1 | LTZ for 3 months and ANA for one month | 3 months | 3 months | ASAS | RF+; anti-CCP +; anti-Jo1+; anti-Ro52 + | High dose corticosteroids (Methylprednisolone, 3500 mg bolus injections, followed by 1 mg/kg/day), Azathioprine (100 mg/day) | Yes (a re-exacerbation was described after the resume of another AIs) |
| Tenti et al. [11] 2019 | Case report | 1 | ANA Duration of the therapy: 6 months | 6 months | 9 months | APS | Antinuclear ab +; aCL IgG and IgM +; aβ2GP1 IgG and IgM+; LAC+ | Enoxaparin 6000 IU for 2 times/day, followed by Warfarin, IVIG therapy (400 mg/kg/day for 5 days, followed by 400 mg/kg/day monthly) and HCQ 200 mg × 2 times/day | N.R. |
| Authors | Country | Study Period | Total Patients | Analyzed Treatment | Reference | Autoimmune Diseases Considered | Incidence Rate Calculation | Estimated Incidence |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [147] 2015 | U.S.A | 1999–2013 | 238,880 | SERM AIs | General population | RA SLE | OR | RA and SERMs: 1.26 for 2–11 months of therapy (95% CI 1.13–1.41); 2.41 for >12 months (95% CI 1.92–3.02;) SLE and SERMs: 1.41 for 2–11 months of therapy (95% CI 1.16–1.71); 2.02 for > 12 months (95% CI 1.29–3.15) RA and AIs: 1.32 for 2–11 months of therapy (95% CI 1.21–1.44); 1.85 for >12 months (95% CI 1.57–2.17). SLE and AIs: 0.84 for 2–11 months of therapy (95% CI 0.70–1.02); 0.77 for >12 months (95% CI 0.50–1.21) |
| Caprioli et al. [148] 2017 | Italy | 2004–2013 | 7533 | Tamoxifen AIs | General population | RA | HR and 95% CI | Incident Rate (95% CI) per 1000 person-years Tamoxifen: 3.01 (1.96 to 4.40); AIs: 3.01 (1.96 to 4.40) |
| Chien et al. [149] 2020 | Taiwan | 2007–2012 | 40,761 | AIs | Tamoxifen users | Any arthritis (including OA, RA and other arthritis); CTS | HR and 95% CI | AIs and any arthritis HR (95% CI): 1.21 (1.09–1.34) AIs and CTS HR (95% CI): 1.68 (1.22–2.32) |
| Wadström et al. [150] 2020 | Sweden | 2006–2016 | 15,921 | Tamoxifen AIs | General population | RA | OR | OR (95% CI): Tamoxifen: 0.86 (0.62 to 1.20) AIs: 0.97 (0.69 to 1.37) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenti, S.; Correale, P.; Cheleschi, S.; Fioravanti, A.; Pirtoli, L. Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020, 21, 5625. https://doi.org/10.3390/ijms21165625
Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L. Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. International Journal of Molecular Sciences. 2020; 21(16):5625. https://doi.org/10.3390/ijms21165625
Chicago/Turabian StyleTenti, Sara, Pierpaolo Correale, Sara Cheleschi, Antonella Fioravanti, and Luigi Pirtoli. 2020. "Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects" International Journal of Molecular Sciences 21, no. 16: 5625. https://doi.org/10.3390/ijms21165625
APA StyleTenti, S., Correale, P., Cheleschi, S., Fioravanti, A., & Pirtoli, L. (2020). Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. International Journal of Molecular Sciences, 21(16), 5625. https://doi.org/10.3390/ijms21165625