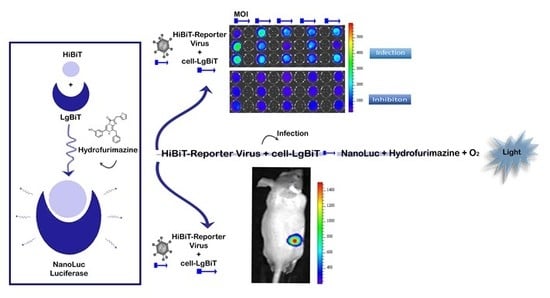
NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice—A Novel in Vivo Imaging Platform
Abstract
:1. Introduction
2. Results
2.1. Expression of HiBiT and Preserved Virulence of the Reporter Virus In Vitro
2.2. Sensitive Longitudinal In Vivo monitoring of HiBiT-Reporter Oncolytic Virus
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture Conditions
4.2. Lentiviral Vector Construction and Cell Transduction
4.3. Reporter Virus Construction
4.4. In Vitro BLI and FLI
4.5. Neutralization Assay
4.6. In Vivo Bioluminescence
4.7. Immunohistochemistry
4.8. Statistical Analysis
4.9. Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelin, A.; Wang, J.; Bell, J.; Boeuf, F. The importance of imaging strategies for pre-clinical and clinical in vivo distribution of oncolytic viruses. Oncolytic Virother. 2018, 7, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, S.M.; McGregor, A. A bright future for bioluminescent imaging in viral research. Future Virol. 2015, 10, 169–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoggins, J.W.; Dorner, M.; Feulner, M.; Imanaka, N.; Murphy, M.Y.; Ploss, A.; Rice, C.M. Dengue reporter viruses reveal viral dynamics in interferon receptor-deficient mice and sensitivity to interferon effectors in vitro. Proc. Natl. Acad. Sci. USA 2012, 109, 14610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luker, G.D.; Leib, D.A. Luciferase real-time bioluminescence imaging for the study of viral pathogenesis. Methods Mol. Biol. 2005, 292, 285–296. [Google Scholar]
- Cook, S.H.; Griffin, D.E. Luciferase Imaging of a neurotropic viral infection in intact animals. J. Virol. 2003, 77, 5333. [Google Scholar] [CrossRef] [Green Version]
- Luker, K.E.; Hutchens, M.; Schultz, T.; Pekosz, A.; Luker, G.D. Bioluminescence imaging of vaccinia virus: Effects of interferon on viral replication and spread. Virology 2005, 341, 284–300. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Fukuhara, T.; Uchida, T.; Ono, C.; Mori, H.; Sato, A.; Fauzyah, Y.; Okamoto, T.; Kurosu, T.; Setoh, Y.X.; et al. Characterization of recombinant flaviviridae viruses possessing a small reporter tag. J. Virol. 2018, 92, e01582-17. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Sharkey, J.; Plagge, A.; Wilm, B.; Murray, P. Multicolour in vivo bioluminescence imaging using a nanoLuc-based BRET reporter in combination with firefly luciferase. Contrast Media Mol. Imaging 2018, 2514796. [Google Scholar] [CrossRef] [Green Version]
- Schwinn, M.K.; Machleidt, T.; Zimmerman, K.; Eggers, C.T.; Dixon, A.S.; Hurst, R.; Hall, M.P.; Encell, L.P.; Binkowski, B.F.; Wood, K.V. CRISPR-mediated tagging of endogenous proteins with a luminescent peptide. ACS Chem. Biol. 2018, 13, 467–474. [Google Scholar] [CrossRef]
- Cai, H.; Liu, M.; Russell, C.J. Directed evolution of an influenza reporter virus to restore replication and virulence and enhance noninvasive bioluminescence imaging in mice. J. Virol. 2018, 92, e00593-18. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Anindita, P.D.; Phongphaew, W.; Carr, M.; Kobayashi, S.; Orba, Y.; Sawa, H. Development of a rapid and quantitative method for the analysis of viral entry and release using a NanoLuc luciferase complementation assay. Virus Res. 2018, 243, 69–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, V.; Moser, L.A.; Poole, D.S.; Mehle, A. Highly sensitive real-time in vivo imaging of an influenza reporter virus reveals dynamics of replication and spread. J. Virol. 2013, 87, 13321–13329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.C.; Walker, J.R.; Park, Y.; Smith, T.P.; Liu, L.X.; Hall, M.P.; Labanieh, L.; Hurst, R.; Wang, D.C.; Encell, L.P.; et al. Novel NanoLuc substrates enable bright two-population bioluminescence imaging in animals. Nat. Methods 2020. [Google Scholar] [CrossRef] [PubMed]
- Farley, D.C.; Brown, J.L.; Leppard, K.N. Activation of the early-late switch in adenovirus type 5 major late transcription unit expression by L4 gene products. J. Virol. 2004, 78, 1782–1791. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, E.A.; Meliopoulos, V.A.; Savage, C.; Livingston, B.; Mehle, A.; Schultz-Cherry, S. Visualizing real-time influenza virus infection, transmission and protection in ferrets. Nat. Commun. 2015, 6, 6378. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Sauthoff, H.; Hu, J.; Maca, C.; Goldman, M.; Heitner, S.; Yee, H.; Pipiya, T.; Rom, W.N.; Hay, J.G. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: Virus persists and spreads systemically at late time points. Hum. Gene Ther. 2004, 14, 425–433. [Google Scholar] [CrossRef]
- Douglas, J.T.; Kim, M.; Sumerel, L.A.; Carey, D.E.; Curiel, D.T. Efficient oncolysis by a replicating adenovirus (Ad) in vivo is critically dependent on tumor expression of primary Ad receptors. Cancer Res. 2001, 61, 813. [Google Scholar]
- de Vrij, J.; Willemsen, R.A.; Lindholm, L.; Hoeben, R.C. Adenovirus-derived vectors for prostate cancer gene therapy. Hum. Gene Ther. 2009, 21, 795–805. [Google Scholar] [CrossRef]
- Marques, R.B.; Erkens-Schulze, S.; de-Ridder, C.M.; Hermans, K.G.; Waltering, K.; Visakorpi, T.; Trapman, J.; Romijin, J.C.; van Weerden, W.M.; Jenster, G. Androgen receptor modifications in prostate cancer cells upon long-termandrogen ablation and antiandrogen treatment. Int. J. Cancer 2005, 117, 221–229. [Google Scholar] [CrossRef]
- Mezzanotte, L.; An, N.; Mol, I.M.; Löwik, C.W.G.M.; Kaijzel, E.L. A new multicolor bioluminescence imaging platform to investigate nf-κb activity and apoptosis in human breast cancer cells. PLoS ONE 2014, 9, e85550. [Google Scholar] [CrossRef] [PubMed]
- Balvers, R.K.; Belcaid, Z.; van den Hengel, S.K.; Kloezeman, J.; de Vrij, J.; Wakimoto, H.; Hoeben, R.C.; Debets, R.; Leenstra, S.; Dirven, C.; et al. Locally-delivered t-cell-derived cellular vehicles efficiently track and deliver adenovirus delta24-rgd to infiltrating glioma. Viruses 2014, 6, 3080–3096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.C.; Zhou, S.; da Costa, L.T.; Yu, J.; Kinzler, K.W.; Vogelstein, B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 1998, 95, 2509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spronken, M.I.; Short, K.R.; Herfst, S.; Bestebroer, T.M.; Vaes, V.P.; van der Hoeven, B.; Koster, A.J.; Kremers, G.J.; Scott, D.P.; Gultyaev, A.P.; et al. Optimisations and challenges involved in the creation of various bioluminescent and fluorescent influenza a virus strains for in vitro and in vivo applications. PLoS ONE 2015, 10, e0133888. [Google Scholar] [CrossRef]
- Falkeholm, L.; Grant, C.A.; Magnusson, A.; Möller, E. Xylene-free method for histological preparation: A multicentre evaluation. Lab. Investig. 2001, 81, 1213–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, N.; Zambito, G.; Dautzenberg, I.J.C.; Cramer, S.J.; Hoeben, R.C.; Lowik, C.; Walker, J.R.; Kirkland, T.A.; Smith, T.P.; van Weerden, W.M.; et al. NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice—A Novel in Vivo Imaging Platform. Int. J. Mol. Sci. 2020, 21, 5863. https://doi.org/10.3390/ijms21165863
Gaspar N, Zambito G, Dautzenberg IJC, Cramer SJ, Hoeben RC, Lowik C, Walker JR, Kirkland TA, Smith TP, van Weerden WM, et al. NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice—A Novel in Vivo Imaging Platform. International Journal of Molecular Sciences. 2020; 21(16):5863. https://doi.org/10.3390/ijms21165863
Chicago/Turabian StyleGaspar, Natasa, Giorgia Zambito, Iris J. C. Dautzenberg, Steve J. Cramer, Rob C. Hoeben, Clemens Lowik, Joel R. Walker, Thomas A. Kirkland, Thomas P. Smith, Wytske M. van Weerden, and et al. 2020. "NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice—A Novel in Vivo Imaging Platform" International Journal of Molecular Sciences 21, no. 16: 5863. https://doi.org/10.3390/ijms21165863
APA StyleGaspar, N., Zambito, G., Dautzenberg, I. J. C., Cramer, S. J., Hoeben, R. C., Lowik, C., Walker, J. R., Kirkland, T. A., Smith, T. P., van Weerden, W. M., de Vrij, J., & Mezzanotte, L. (2020). NanoBiT System and Hydrofurimazine for Optimized Detection of Viral Infection in Mice—A Novel in Vivo Imaging Platform. International Journal of Molecular Sciences, 21(16), 5863. https://doi.org/10.3390/ijms21165863