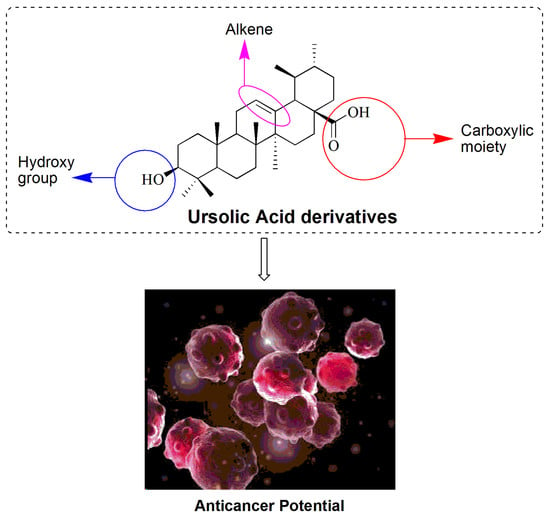
Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update
Abstract
:1. Introduction
2. An overview of Pharmacokinetics of UA and Its Derivatives
Clinical Trials of UA
3. Chemistry of UA
3.1. UA Derivatives as an Anticancer Agent
3.1.1. Modification of the Carboxylic Moiety (C-28)
3.1.2. Modification of both β-hydroxy (C-3) and Carboxylic Moiety (C-28)
3.1.3. Modification of β-Hydroxy (C-3 Position)
3.1.4. Modification of Miscellaneous Groups
4. Insights and Future Directions
Funding
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| UA | Ursolic acid |
| P53 | Tumor protein p53 |
| Wnt | Wnt/β-catenin pathways |
| Ras | Retrovirus-associated DNA sequences |
| TRAIL | TNF-related apoptosis-inducing ligand |
| STAT3 | Signal transducers and activators of transcription |
| PK | Pharmacokinetic |
| UV | Ultraviolet |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| DMF | Dimethylformamide |
| MTT | Dye compound 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay |
| SRB | sulforhodamine B assay |
| PARP-1 | Poly [ADP-ribose] polymerase 1 |
| NF-kB | Nuclear factor kappa-B |
| DMAP | 4-Dimethylaminopyridine |
| DCM | Dichloromethane |
| THF | Tetrahydrofuran |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMSO | Dimethyl sulfoxide |
| FROS | Reactive oxygen species |
| MEK | Mitogen-activated extracellular signal-regulated kinase |
References
- Dewangan, J.; Srivastava, S.; Mishra, S.; Divakar, A.; Kumar, S.; Rath, S.K. Salinomycin inhibits breast cancer progression via targeting HIF-1α/VEGF mediated tumor angiogenesis in vitro and in vivo. Biochem. Pharmacol. 2019, 164, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Made, F.; Wilson, K.; Jina, R.; Tlotleng, N.; Jack, S.; Ntlebi, V.; Kootbodien, T. Distribution of cancer mortality rates by province in South Africa. Cancer Epidemiol. 2017, 51, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.C.; Anil Kumar, N.V.; Thakur, G. Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. Eur. J. Med. Chem. 2019, 177, 76–104. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Jovanovi, K.K.; Mojovi, M.; Savi, A.G. New and Highly Potent Antitumor Natural Products from Marine-Derived Fungi: Covering the Period from 2003 to 2012. Curr. Top. Med. Chem. 2013, 13, 2745–2766. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, P.S. Plant Derived Anticancer Agents: A Review. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 293–308. [Google Scholar]
- Pejin, B.; Glumac, M.A. brief review of potent anti-CNS tumourics from marine sponges: Covering the period from 1994 to 2014. Nat. Prod. Res. 2018, 32, 375–384. [Google Scholar] [CrossRef]
- Calcabrini, C.; Catanzaro, E.; Bishayee, A.; Turrini, E.; Fimognari, C. Marine Sponge Natural Products with Anticancer Potential: An Updated Review. Mar. Drugs. 2017, 15, 310. [Google Scholar] [CrossRef] [Green Version]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Hu, J.; Wang, H.; Xu, F.; Bian, Q. Application of natural products derivatization method in the design of targeted anticancer agents from 2000 to 2018. Bioorganic Med. Chem. 2019, 27, 115150. [Google Scholar] [CrossRef]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules 2018, 23, 2300. [Google Scholar] [CrossRef] [Green Version]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Gonçalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Sudhagar, S.; Sarathkumar, B.; Lakshmi, B.S. EGFR inhibition by pentacyclic triterpenes exhibit cell cycle and growth arrest in breast cancer cells. Life Sci. 2014, 95, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hu, Y.L.; Wang, H. Ursolic acid inhibits breast cancer growth by inhibiting proliferation, inducing autophagy and apoptosis, and suppressing inflammatory responses via the PI3K/AKT and NF-κB signaling pathways in vitro. Exp. Ther. Med. 2017, 14, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarowicz, R.; Pegg, R.B. Inhibition of proliferation of human carcinoma cell lines by phenolic compounds from a bearberry-leaf crude extract and its fractions. J. Funct. Foods. 2013, 5, 660–667. [Google Scholar] [CrossRef]
- Hassan, H.M.; Jiang, Z.H.; Asmussen, C.; McDonald, E.; Qin, W. Antibacterial activity of northern Ontario medicinal plant extracts. Can. J. Plant Sci. 2014, 94, 417–424. [Google Scholar] [CrossRef]
- Guinda, A.; Rada, M.; Delgado, T.; Castellano, J.M. Pentacyclic triterpenic acids from Argania spinosa. Eur. J. Lipid Sci. Technol. 2011, 113, 231–237. [Google Scholar] [CrossRef]
- Guinda, Á.; Rada, M.; Delgado, T.; Gutiérrez-Adánez, P.; Castellano, J.M. Pentacyclic triterpenoids from olive fruit and leaf. J. Agric. Food Chem. 2010, 58, 9685–9691. [Google Scholar] [CrossRef]
- García-Morales, G.; Huerta-Reyes, M.; González-Cortazar, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Silva-García, R.; Román-Ramos, R.; Aguilar-Rojas, A. Anti-inflammatory, antioxidant and anti-acetylcholinesterase activities of Bouvardia ternifolia: Potential implications in Alzheimer’s disease. Arch. Pharm. Res. 2015, 38, 1369–1379. [Google Scholar] [CrossRef]
- Figueroa-Suárez, M.Z.; González, C.J.; Cardoso-Taketa, A.T.; Del Carmen Gutiérrez Villafuerte, M.; Rodríguez-López, V. Anti-inflammatory and antihistaminic activity of triterpenoids isolated from Bursera cuneata (Schldl.) Engl. J. Ethnopharmacol. 2019, 238, 111786. [Google Scholar] [CrossRef]
- Yu, F.; Thamm, A.M.K.; Reed, D.; Villa-Ruano, N.; Quesada, A.L.; Gloria, E.L.; Covello, P.; De, L.V. Functional characterization of amyrin synthase involved in ursolic acid biosynthesis in Catharanthus roseus leaf epidermis. Phytochemistry 2013, 91, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.H.; Park, H.Y.; Kim, J.Y.; Jeong, I.Y.; Lee, M.K.; Seo, K.-I. Apoptotic action of ursolic acid isolated from Corni fructus in RC-58T/h/SA#4 primary human prostate cancer cells. Bioorganic Med. Chem. Lett. 2010, 20, 6435–6438. [Google Scholar]
- Zar, P.P.K.; Yano, S.; Sakao, K.; Hashimoto, F.; Nakano, T.; Fujii, M.; Hou, D.X. In vitro anticancer activity of loquat tea by inducing apoptosis in human leukemia cells. Biosci. Biotechnol. Biochem. 2014, 78, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhao, C.; Zhu, Q.; Katakura, Y.; Tanaka, H.; Ohnuki, K.; Shimizu, K. Ursolic Acid Isolated from the Leaves of Loquat (Eriobotrya japonica) Inhibited Osteoclast Differentiation through Targeting Exportin 5. J. Agric. Food Chem. 2019, 67, 3333–3340. [Google Scholar] [CrossRef]
- Tan, H.; Sonam, T.; Shimizu, K. The potential of triterpenoids from loquat leaves (Eriobotrya japonica) for prevention and treatment of skin disorder. Int. J. Mol. Sci. 2017, 18, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuraoka-Oliveira, Â.M.; Radai, J.A.S.; Leitão, M.M.; Lima Cardoso, C.A.; Silva-Filho, S.E.; Leite, K.C.A. Anti-inflammatory and anti-arthritic activity in extract from the leaves of Eriobotrya japonica. J. Ethnopharmacol. 2020, 249, 112418. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.S.; Yuan, Y.; Song, G.; Lin, S.Q. Inhibitory effect of ursolic acid and oleanolic acid from Eriobotrya fragrans on A549 cell viability in vivo. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.A.; Patinha, D.J.S.; Sousa, G.D.A.; Villaverde, J.J.; Silva, C.M.; Freire, C.S.R. Eucalyptus biomass residues from agro-forest and pulping industries as sources of high-value triterpenic compounds. Cellul. Chem. Technol. 2011, 45, 475–481. [Google Scholar]
- Madmanang, S.; Cheyeng, N.; Heembenmad, S.; Mahabusarakam, W.; Saising, J.; Seeger, M.; Chusri, S.; Chakthong, S. Constituents of Fagraea fragrans with Antimycobacterial Activity in Combination with Erythromycin. J. Nat. Prod. 2016, 79, 767–774. [Google Scholar] [CrossRef]
- Palu, D.; Bighelli, A.; Casanova, J.; Paoli, M. Identification and quantitation of ursolic and oleanolic acids in ilex aquifolium l. Leaf extracts using 13C and 1H-NMR spectroscopy. Molecules 2019, 24, 4413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójciak-Kosior, M.; Sowa, I.; Kocjan, R.; Nowak, R. Effect of different extraction techniques on quantification of oleanolic and ursolic acid in Lamii albi flos. Ind. Crops Prod. 2013, 44, 373–377. [Google Scholar] [CrossRef]
- Jamal, M.; Amir, M.; Ali, Z.; Mujeeb, M. A comparative study for the extraction methods and solvent selection for isolation, quantitative estimation and validation of ursolic acid in the leaves of Lantana camara by HPTLC method. Futur. J. Pharm. Sci. 2018, 4, 229–233. [Google Scholar] [CrossRef]
- Kazmi, I.; Rahman, M.; Afzal, M.; Gupta, G.; Saleem, S.; Afzal, O.; Shaharyar, M.A.; Nautiyal, U.; Ahmed, S.; Anwar, F. Anti-diabetic potential of ursolic acid stearoyl glucoside: A new triterpenic gycosidic ester from Lantana camara. Fitoterapia 2012, 83, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Afzal, M.; Ali, B.; Damanhouri, Z.A.; Ahmaol, A.; Anwar, F. Anxiolytic potential of ursolic acid derivative-a stearoyl glucoside isolated from Lantana camara L. (verbanaceae). Asian Pac. J. Trop. Med. 2013, 6, 433–437. [Google Scholar] [CrossRef]
- Gilabert, M.; Marcinkevicius, K.; Andujar, S.; Schiavone, M.; Arena, M.E.; Bardón, A. Sesqui- and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine 2015, 22, 77–85. [Google Scholar] [CrossRef]
- Xia, E.Q.; Yu, Y.Y.; Xu, X.R.; Deng, G.F.; Guo, Y.J.; Bin Li, H. Ultrasound-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Ultrason. Sonochem. 2012, 19, 772–776. [Google Scholar] [CrossRef]
- Xia, E.Q.; Wang, B.W.; Xu, X.R.; Zhu, L.; Song, Y.; Li, H.B. Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum ait. Int. J. Mol. Sci. 2011, 12, 5319–5329. [Google Scholar] [CrossRef]
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Noshita, T.; Kidachi, Y.; Umetsu, H.; Hayashi, M.; Komiyama, K.; Funayama, S.; Ryoyama, K. Isolation of ursolic acid from apple peels and its specific efficacy as a potent antitumor agent. J. Heal. Sci. 2008, 54, 654–660. [Google Scholar] [CrossRef] [Green Version]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth (family Lamiaceae). South Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Ahmad, A.; Abuzinadah, M.F.; Alkreathy, H.M.; Banaganapalli, B.; Mujeeb, M. Ursolic acid rich ocimum sanctum L leaf extract loaded nanostructured lipid carriers ameliorate adjuvant induced arthritis in rats by inhibition of COX-1, COX-2, TNF-α and IL-1: Pharmacological and docking studies. PLoS ONE 2018, 13, e0193451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flegkas, A.; Milosević Ifantis, T.; Barda, C.; Samara, P.; Tsitsilonis, O.; Skaltsa, H. Antiproliferative Activity of (-)-Rabdosiin Isolated from Ocimum sanctum L. Medicines 2019, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jothie, R.E.; Illuri, R.; Bethapudi, B.; Anandhakumar, S.; Bhaskar, A.; Chinampudur, V.C.; Mundkinajeddu, D.; Agarwal, A. Activity of Ocimum sanctum: Possible Effects on Hypothalamic-Pituitary-Adrenal Axis. Phyther. Res. 2016, 30, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001, 16, S28–S37. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.A.; Ibrahim, N.A.; Mohammed, M.M.D.; El-Hawary, S.; Refaat, E.A. The potential chemo preventive effect of ursolic acid isolated from Paulownia tomentosa, against N-diethylnitrosamine: Initiated and promoted hepatocarcinogenesis. Heliyon 2019, 5, e01769. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Hu, Y.; Li, J.; Shi, K.; Shen, Y.; Zhu, B.; Wang, G.X. Ursolic acid from Prunella vulgaris L. efficiently inhibits IHNV infection in vitro and in vivo. Virus Res. 2019, 273, 197741. [Google Scholar] [CrossRef]
- Kim, H.I.; Quan, F.; Kim, J.; Lee, N.; Kim, H.; Jo, S.; Lee, C.M.; Jang, D.; Inn, K.S. Inhibition of estrogen signaling through depletion of estrogen receptor alpha by ursolic acid and betulinic acid from Prunella vulgaris var. lilacina. Biochem. Biophys. Res. Commun. 2014, 451, 282–287. [Google Scholar] [CrossRef]
- Xu, C.; Liao, Y.; Fang, C.; Tsunoda, M.; Zhang, Y.; Song, Y.; Deng, S. Simultaneous Analysis of Ursolic Acid and Oleanolic Acid in Guava Leaves Using QuEChERS-Based Extraction Followed by High-Performance Liquid Chromatography. J. Anal. Methods Chem. 2017, 2017, 2984562. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C.; Huang, T.C. Optimisation of an ultrasound-assisted extraction followed by RP-HPLC separation for the simultaneous determination of oleanolic acid, ursolic acid and oridonin content in Rabdosia rubescens. Phytochem. Anal. 2012, 23, 627–636. [Google Scholar] [CrossRef]
- MacHado, D.G.; MacHado, D.G.; Neis, V.B.; Balen, G.O.; Colla, A.; Cunha, M.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Prediger, R.D.; Rodrigues, A.L.S. Antidepressant-like effect of ursolic acid isolated from Rosmarinus officinalis L. in mice: Evidence for the involvement of the dopaminergic system. Pharmacol. Biochem. Behav. 2012, 103, 204–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, Â.M.C.; Ferreira, D.A. Antibacterial and Antioxidant Activities of Ursolic Acid. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Siwu, E.R.O.; Nozaki, S.; Watanabe, Y.; Tanaka, K.; Fukase, K. Ursolic acid derivatives from Bangladeshi medicinal plant, Saurauja roxburghii: Isolation and cytotoxic activity against A431 and C6 glioma cell lines. Phytochem. Lett. 2011, 4, 287–291. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer activities of thymus vulgaris L. In experimental breast carcinoma in vivo and in vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, V.; Rojas, F.; Tedesco, V.; Giusiano, G.; Angiolella, L. Chemical characterization and antifungal activity of Origanum vulgare, Thymus vulgaris essential oils and carvacrol against Malassezia furfur. Nat. Prod. Res. 2019, 33, 3273–3277. [Google Scholar] [CrossRef]
- Abu-Gharbieh, E.; Shehab, N.G.; Almasri, I.M.; Bustanji, Y. Antihyperuricemic and xanthine oxidase inhibitory activities of Tribulus arabicus and its isolated compound, ursolic acid: In vitro and in vivo investigation and docking simulations. PLoS ONE 2018, 13, e0202572. [Google Scholar] [CrossRef]
- Ksiksi, T.; Palakkott, A.R.; Ppoyil, S.B.T. Tribulus arabicus and Tribulus macropterus are Comparable to Tribulus terrestris: An Antioxidant Assessment. Curr. Bioact. Compd. 2017, 13, 82–87. [Google Scholar] [CrossRef]
- Sharifiyan, F.; Mirjalili, S.A.; Fazilati, M.; Poorazizi, E.; Habibollahi, S. Variation of ursolic acid content in flowers of ten Iranian pomegranate (Punica granatum L.) cultivars. BMC Chem. 2019, 13, 80. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Zhang, L.; Cheng, N.; Jia, M.; Zhang, Y. Extraction optimization of oleanolic and ursolic acids from pomegranate (Punica granatum L.) flowers. Food Bioprod. Process. 2014, 92, 321–327. [Google Scholar] [CrossRef]
- Jung, T.Y.; Pham, T.N.N.; Umeyama, A.; Shoji, N.; Hashimoto, T.; Lee, J.; Takei, M. Ursolic acid isolated from Uncaria rhynchophylla activates human dendritic cells via TLR2 and/or TLR4 and induces the production of IFN-γ by CD4+ naïve T cells. Eur. J. Pharmacol. 2010, 643, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W.Z.; Yao, C.L.; Feng, R.H.; Yang, M.; Guo, D.; Wu, W.Y. New triterpenic acids from Uncaria rhynchophylla: Chemistry, NO-inhibitory activity, and tandem mass spectrometric analysis. Fitoterapia 2014, 96, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chandramu, D.; Rao, D.M.; Krupadanam, G.I.D.; Reddy, V.R. Isolation, Characterization and Biological Activity of Betulinic Acid and Ursolic Acid from Vitex negundo L. Phyther. Res. 2003, 134, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kitamura, K.; Irie, K.; Naruse, S.; Matsuura, T.; Uemae, T.; Taira, S.; Ohigashi, H.; Murakami, S.; Takahashi, M.; et al. Triterpenoids isolated from Ziziphus jujuba enhance glucose uptake activity in skeletal muscle cells. J. Nutr. Sci. Vitaminol. 2017, 63, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Yin, R.; Li, T.; Tian, J.X.; Xi, P.; Liu, R.H. Ursolic acid, a potential anticancer compound for breast cancer therapy. Crit. Rev. Food Sci. Nutr. 2018, 58, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PloS ONE. 2012, 7, e31318. [Google Scholar] [CrossRef] [Green Version]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Castro, A.J.; Frederico, M.J.; Cazarolli, L.H.; Mendes, C.P.; Bretanha, L.C.; Schmidt, É.C.; Bouzon, Z.L.; de Medeiros Pinto, V.A.; da Fonte Ramos, C.; Pizzolatti, M.G.; et al. The mechanism of action of ursolic acid as insulin secretagogue and insulinomimetic is mediated by cross-talk between calcium and kinases to regulate glucose balance. Biochim. Biophys. Acta. 2015, 1850, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, M.K.; Dai, X.; Prem, A.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [Green Version]
- Angeles, M.L.; Navin, R.; Kim, S.M. Therapeutic Interventions Using Ursolic Acid for Cancer Treatment. Med. Chem. 2016, 6, 339–344. [Google Scholar]
- Achiwa, Y.; Hasegawa, K.; Udagawa, Y.; Achiwa, Y.; Hasegawa, K.; Udagawa, Y. Effect of Ursolic Acid on MAPK in Cyclin D1 Signaling and RING-Type E3 Ligase (SCF E3s) in Two Endometrial Cancer Cell Lines Effect of Ursolic Acid on MAPK in Cyclin D1 Signaling and RING-Type E3 Ligase (SCF E3s) in Two Endometrial. Nutr. Cancer 2013, 65, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Achiwa, Y.; Hasegawa, K.; Udagawa, A. Regulation of the Phosphatidylinositol 3-Kinase-Akt and the Mitogen-Activated Protein Kinase Pathways by Ursolic Acid in Human Endometrial Cancer Cells Yumiko. Biosci. Biotechnol. Biochem. 2007, 71, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.; Yang, X. Ursolic acid inhibits growth and induces apoptosis in gemcitabine-resistant human pancreatic cancer via the JNK and PI3K/Akt/NF- κ B pathways. Oncol. Rep. 2012, 28, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Yadav, V.R.; Sung, B.; Gupta, S.C.; Tyagi, A.K. Ursolic acid inhibits the growth of human pancreatic cancer and enhances the antitumor potential of gemcitabine in an orthotopic mouse model through suppression of the inflammatory microenvironment. Oncotarget 2016, 7, 13182. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, C.Y.; Tsai, C.W.; Yin, M.C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. Vitr. 2011, 25, 1274–1280. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.G.; Lee, J.; Shin, J.; Harikishore, A.; Jung, H.Y.; Kim, Y.S.; Lyu, H.N.; Oh, E.; Baek, N.I.; et al. Ursolic acid exerts anti-cancer activity by suppressing vaccinia-related kinase 1-mediated damage repair in lung cancer cells. Sci. Rep. 2015, 5, 14570. [Google Scholar] [CrossRef]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Rajendran, P.; Li, F.; Nema, T.; Vali, S.; Abbasi, T.; Kapoor, S.; Sharma, A.; Kumar, A.P.; Ho, P.C.; et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011, 89, 713–727. [Google Scholar] [CrossRef]
- Gai, W.T.; Yu, D.P.; Wang, X.S.; Wang, P.T. Anti-cancer effect of ursolic acid activates apoptosis through ROCK/PTEN mediated mitochondrial translocation of cofilin-1 in prostate cancer. Oncol. Lett. 2016, 12, 2880–2885. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Wang, B.; Xiang, J. Effects of ursolic acid on the proliferation and apoptosis of human ovarian cancer cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Lu, Z.; Yuet, W.J.; Zhou, L.L.; Fung, K.P.; Wu, P.; Wu, S. Ursolic acid induces doxorubicin-resistant HepG2 cell death via the release of apoptosis-inducing factor. Cancer Lett. 2010, 298, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Li, P.; Jin, F.; Yao, C.; Zhang, G.; Zang, T.; Ai, X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell. Signal. 2013, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, G.; Zhang, K.; Zhou, Y.; Li, X.; Xu, W.; Zhang, H.; Shao, Y.; Zhang, Z.; Sun, W. Cyclooxygenase-2 mediated synergistic effect of ursolic acid in combination with paclitaxel against human gastric carcinoma. Oncotarget 2017, 8, 92770–92777. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Moon, A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, L.; Cheng, Y.; Liu, Y. Ursolic Acid Improves Liver Transplantation and Inhibits Apoptosis in Miniature Pigs Using Donation after Cardiac Death. Cell. Physiol. Biochem. 2017, 43, 331–338. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors. J. Biol. Chem. 2016, 291, 16924. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lin, C.; Hua, C.; Jou, Y.; Liao, C.; Chang, Y.; Wan, L.; Huang, S.; Hour, M.; Lin, C. Cis-3-O-p-hydroxycinnamoyl ursolic acid induced ROS-dependent p53-mediated mitochondrial apoptosis in oral cancer cells. Biomol. Ther. 2019, 27, 54–62. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Dong, Y. Ursolic acid nanoparticles inhibit cervical cancer growth in vitro and in vivo via apoptosis induction. Int. J. Oncol. 2017, 50, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid: Suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.H.; Song, G.; Kim, D.; Jeong, Y.; Liu, K.; Chung, Y.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Tian, D.; Liu, Y.; Nian, W.; Zou, X.; Chen, Q.; Zhou, L.; Deng, Z.; He, B. Ursolic acid inhibits proliferation and induces apoptosis by inactivating Wnt/β-catenin signaling in human osteosarcoma cells. Int. J. Oncol. 2016, 49, 1973–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jin, H.; Meng, R.Y.; Kim, D.Y.; Liu, Y.C.; Chai, O.H.; Park, B.H.; Kim, S. Activating hippo pathway via Rassf1 by ursolic acid suppresses the tumorigenesis of gastric cancer. Int. J. Mol. Sci. 2019, 20, 4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Huang, R.; Zhang, J.; Guo, T.; Zhang, M.; Huang, X.; Zhang, B.; Liao, Z.; Sun, J.; Wang, H. Discovery of antitumor ursolic acid long-chain diamine derivatives as potent inhibitors of NF-κB. Bioorg. Chem. 2018, 79, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Park, J.W. Ursolic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Biochim. Biophys. Acta. 2013, 1833, 723–730. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Kwang, S.A.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhao, C.; Jou, D.; Lü, J.; Zhang, C.; Lin, L.; Lin, J. Ursolic acid inhibits the growth of colon cancer-initiating cells by targeting STAT3. Anticancer Res. 2013, 33, 4279–4284. [Google Scholar]
- Liu, T.; Ma, H.; Shi, W.; Duan, J.; Wang, Y.; Zhang, C.; Li, C.; Lin, J.; Li, S.; Lv, J.; et al. Inhibition of STAT3 signaling pathway by ursolic acid suppresses growth of hepatocellular carcinoma. Int. J. Oncol. 2017, 51, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Jinhua, W. Ursolic acid: Pharmacokinetics process in vitro and in vivo, a mini review. Arch. Pharm. 2019, 352, 1800222. [Google Scholar] [CrossRef]
- Eloy, J.O.; Saraiva, J.; De Albuquerque, S.; Marchetti, J.M. Preparation, Characterization and evaluation of the in vivo trypanocidal activity of ursolic acid-loaded solid dispersion with poloxamer 407 and sodium caprate. J. Pharm. Sci. 2015, 51, 101–109. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Wang, A.; Zhou, X.; Zheng, Y.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, S.; Prasad, N.R. Effect of ursolic acid, a triterpenoid antioxidant, on ultraviolet-B radiation-induced cytotoxicity, lipid peroxidation and DNA damage in human lymphocytes. Chem. Biol. Interact. 2008, 176, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Martin-Aragón, S.; De Las Heras, B.; Sanchez-Reus, M.I.; Benedi, J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp. Toxicol. Pathol. 2001, 53, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, W.; Zhang, J.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of ursolic acid against carbapenem-resistant Klebsiella pneumoniae. J. Antibiot. 2020, 73, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Li, X.; Shen, L.; Wang, T.; Liu, M.; Zhang, J.; Yang, M.; Li, X.; Cai, C. Antibacterial and antibiofilm activity of ursolic acid against carbapenem-resistant Enterobacter cloacae. J. Biosci. Bioeng. 2020, 125, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Spivak, A.Y.; Khalitova, R.R.; Nedopekina, D.A.; Gubaidullin, R.R. Antimicrobial properties of amine- and guanidine-functionalized derivatives of betulinic, ursolic and oleanolic acids: Synthesis and structure/activity evaluation. Steroids 2020, 154, 108530. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Oloyede, H.O.B.; Ajiboye, H.O.; Salawu, M.O.; Ajiboye, T.O. Influence of oxidative stress on the antibacterial activity of betulin, betulinic acid and ursolic acid. Microb. Pathog. 2017, 111, 338–344. [Google Scholar] [CrossRef]
- Hedyotis, L. Hepatoprotective and Antibacterial Activity of Ursolic Acid Extracted from. Bangladesh J. Sci. Ind. Res. 2010, 45, 27–34. [Google Scholar]
- Shu, C.; Zhao, H.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Antifungal efficacy of ursolic acid in control of Alternaria alternata causing black spot rot on apple fruit and possible mechanisms involved. Sci. Hortic. 2019, 256, 108636. [Google Scholar] [CrossRef]
- Mahlo, S.M.; McGaw, L.J.; Eloff, J.N. Antifungal activity and cytotoxicity of isolated compounds from leaves of Breonadia salicina. J. Ethnopharmacol. 2013, 148, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Zahari, R.; Halimoon, N.; Ahmad, M.F.; Ling, S.K. Antifungal Compound Isolated from Catharanthus roseus L. (Pink) for Biological Control of Root Rot Rubber Diseases. Int. J. Anal. Chem. 2018, 2018, 8150610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaik, A.B.; Ahil, S.B.; Govardhanam, R.; Senthi, M.; Khan, R.; Sojitra, R.; Kumar, S.; Srinivas, A. Antifungal Effect and Protective Role of Ursolic Acid and Three Phenolic Derivatives in the Management of Sorghum Grain Mold Under Field Conditions. Chem. Biodivers. 2016, 13, 1158–1164. [Google Scholar] [CrossRef]
- Wang, X.; Habib, E.; Leõn, F.; Radwan, M.M.; Tabanca, N.; Gao, J.; Wedge, D.E.; Cutler, S.J. Antifungal metabolites from the roots of diospyros virginiana by overpressure layer chromatography. Chem. Biodivers. 2011, 8, 2331–2340. [Google Scholar] [CrossRef]
- Innocente, A.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C.B. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef]
- Luciano, J.H.S.; Lima, M.A.S.; Silveira, E.R.; Vasconcelos, I.M.; Fernandes, G.S.; De Souza, E.B. Antifungal iridoids, triterpenes and phenol compounds from alibertia myrciifolia sprunge EX.schum. Quim. Nova 2010, 33, 292–294. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Al-Yousef, H.M.; Siddiqui, N.A.; Alhowiriny, T.A.; Alqasoumi, S.I.; Amina, M.; Hassan, W.H.B.; Abdelaziz, S.; Abdalla, R.H. Anticancer activity and concurrent analysis of ursolic acid, β-sitosterol and lupeol in three different Hibiscus species (aerial parts) by validated HPTLC method. Saudi Pharm. J. 2018, 26, 1060–1067. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Soon, C.Y.; Tan, J.B.L.; Wong, S.K.; Hui, Y.W. Ursolic acid: An overview on its cytotoxic activities against breast and colorectal cancer cells. J. Integr. Med. 2019, 17, 155–160. [Google Scholar] [CrossRef]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.H.; Shin, Y.C.; Ko, S.G. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch. Pharm. Res. 2011, 34, 1363–1372. [Google Scholar] [CrossRef]
- Wang, J.S.; Ren, T.N.; Xi, T. Ursolic acid induces apoptosis by suppressing the expression of FoxM1 in MCF-7 human breast cancer cells. Med. Oncol. 2012, 29, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Wu, C.H.; Yen, G.C. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 2010, 54, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Nie, M.; Wang, W.; Liu, Z.; Chen, C.; Chen, H.; Liu, R.; Baloch, Z.; Ma, K. Novel synthetic ursolic acid derivative inhibits growth and induces apoptosis in breast cancer cell lines. Oncol. Lett. 2018, 15, 2323–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.P.; Zhang, K.; Lu, Y.J.; He, P.; Zhao, S.Q. In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2014, 80, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Ávila, R.; Flores-Morales, V.; Paoli, P.; Camici, G.; Ramírez-Espinosa, J.J.; Cerón-Romero, L.; Navarrete-Vázquez, G.; Hidalgo-Figueroa, S.; Yolanda Rios, M.; Villalobos-Molina, R.; et al. Ursolic acid derivatives as potential antidiabetic agents: In vitro, in vivo, and in silico studies. Drug Dev. Res. 2018, 79, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Alkreathy, H.M.; Ahmad, A. Catharanthus roseus Combined with Ursolic Acid Attenuates Streptozotocin-Induced Diabetes through Insulin Secretion and Glycogen Storage. Oxid. Med. Cell. Longev. 2020, 2020, 8565760. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of glycosidase by ursolic acid: In vitro, in vivo and in silico study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Uzaşçı, S.; Dirmenci, T.; Erim, F.B. α-Glucosidase enzyme inhibitory effects and ursolic and oleanolic acid contents of fourteen Anatolian Salvia species. J. Pharm. Biomed. Anal. 2018, 155, 284–287. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, H.; Sui, Z.; Jing, F.; Quan, X.; Zhao, W.; Liu, G. Ursolic acid exhibits anti-inflammatory effects through blocking TLR4-MyD88 pathway mediated by autophagy. Cytokine 2019, 123, 154726. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, J.K.; Jeong, N.; Yoo, J.; Ha, Y.S.; Lee, B.; Choi, H.; Park, P.; Shin, T.; Kwon, T.K.; et al. Anti-inflammatory effects of ursolic acid-3-acetate on human synovial fibroblasts and a murine model of rheumatoid arthritis. Int. Immunopharmacol. 2017, 49, 118–125. [Google Scholar] [CrossRef]
- Rai, S.N.; Zahra, W.; Singh, S.S.; Birla, H.; Keswani, C.; Dilnashin, H.; Rathore, A.S.; Singh, R.; Singh, R.K.; Singh, S.P. Anti-inflammatory Activity of Ursolic Acid in MPTP-Induced Parkinsonian Mouse Model. Neurotox. Res. 2019, 36, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antioxidant and Anti-inflammatory Mechanisms of Neuroprotection by Ursolic Acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.A.; Royo, V.A.; Ferreira, D.S.; Crotti, A.E.; e Silva, M.L.; Carvalho, J.C.; Bastos, J.K.; Cunha, W.R. In vivo analgesic and anti-inflammatory activities of ursolic acid and oleanoic acid from Miconia albicans (Melastomataceae). Z. Naturforsch C. 2006, 61, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Tohmé, M.J.; Giménez, M.C.; Peralta, A.; Colombo, M.I.; Delgui, L.R. Ursolic acid: A novel antiviral compound inhibiting rotavirus infection in vitro. Int. J. Antimicrob. Agents. 2019, 54, 601–609. [Google Scholar] [CrossRef]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorganic Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef] [PubMed]
- Babalola, I.T.; Shode, F.O. Ubiquitous Ursolic Acid: A Potential Pentacyclic Triterpene Natural Product. J. Pharmacogn. Phytochem. 2013, 2, 214–222. [Google Scholar]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [Green Version]
- Hua, S.X.; Huang, R.Z.; Ye, M.Y.; Pan, Y.M.; Yao, G.; Zhang, Y. Design, synthesis and in vitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur. J. Med. Chem. 2015, 95, 435–452. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zhang, H.; Yue, J.; Sun, Y.; Zhang, X.; Zhao, Y. Synthesis of triterpenoid derivatives and their anti-tumor and anti-hepatic fibrosis activities. Nat. Prod. Res. 2020, 34, 766–772. [Google Scholar] [CrossRef]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- De Angel, R.E.; Smith, S.M.; Glickman, R.D.; Perkins, S.N.; Hursting, S.D. Antitumor Effects of Ursolic Acid in a Mouse Model of Postmenopausal Breast Cancer. Nutr. Cancer 2010, 62, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.; Macdonalda, C.; Walligb, M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz [a] anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996, 104, 43–48. [Google Scholar] [CrossRef]
- Alzate, M.A.H.; Filho, M.A.F.N.; Franchin, T.B.; Jonata, M.A.H.; Oliveira, A.D.J.; Candido, C.D.; Furini, J.; Albuquerque, S.D.; Gonçalves, R. Kinetic disposition of ursolic acid in rats. Pharm. Biomed. Res. 2018, 4, 25–31. [Google Scholar]
- Liao, Q.; Yang, W.; Jia, Y.; Chen, X.; Gao, Q.; Bi, K. LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional Chinese medicinal preparation Lu-Ying extract. Yakugaku Zasshi 2005, 125, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.; Wang, B.; Wei, C.; Yuan, G.; Bu, F.; Guo, R. Determination of glycyrrhetic acid in human plasma by HPLC-MS method and investigation of its pharmacokinetics. J. Clin. Pharm. Ther. 2008, 33, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, S. Determination of asiatic acid in beagle dog plasma after oral administration of Centella asiatica extract by precolumn derivatization RP-HPLC. J. Chromatogr. B J. 2009, 877, 477–481. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Liu, Y.; Wang, Q.; Xing, S.; Li, L.; Wang, L.; Liu, L.; Gao, D. Ursolic acid liposomes with chitosan modification: Promising antitumor drug delivery and efficacy. Mater. Sci. Eng. C. 2017, 71, 1231–1240. [Google Scholar] [CrossRef]
- Yoon, Y.; Woong, J.; Kim, J.; Kim, Y.; Ho, K. Discovery of ursolic acid prodrug (NX-201): Pharmacokinetics and in vivo antitumor effects in PANC-1 pancreatic cancer. Bioorg. Med. Chem. Lett. 2016, 26, 5524–5527. [Google Scholar] [CrossRef]
- Zhu, Z.; Qian, Z.; Yan, Z.; Zhao, C.; Wang, H. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int. J. Nanomed. 2013, 8, 129–136. [Google Scholar]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.A. Phase I Trial to Evaluate the Multiple-Dose Safety and Antitumor Activity of Ursolic Acid Liposomes in Subjects with Advanced Solid Tumors. BioMed Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Qian, Z.Z.; Zhang, H.L.; Qiu, L.H.; Song, S.; Zhao, J.; Wang, P.; Hao, X.S.; Wang, H.Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Both, D.M.; Goodtzova, K.; Yarosh, D.B.; Brown, D.A. Liposome-encapsulated ursolic acid increases ceramides and collagen in human skin cells. Arch. Dermatol. Res. 2002, 293, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Singh, H.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, X.; Jingyang, E.L. Synthesis of novel oleanolic acid and ursolic acid in C-28 position derivatives as potential anticancer agents. Arch. Pharm. Res. 2017, 40, 458–468. [Google Scholar] [CrossRef]
- Chi, K.; Wei, Z.; Wang, K.; Wu, J.; Chen, W.; Jin, X.; Piao, H. Design, synthesis, and evaluation of novel ursolic acid derivatives as HIF-1 a inhibitors with anticancer potential. Bioorg. Chem. 2017, 75, 157–169. [Google Scholar] [CrossRef]
- Liu, M.; Yang, S.; Hu, L.J.D.; Xue, W.; Eb, A.O. Synthesis and evaluation as potential antitumor agents of novel ursolic acid derivatives. Med. Chem. Res. 2016, 2267–2279. [Google Scholar] [CrossRef]
- Wiemann, J.; Heller, L.; Csuk, R. Targeting cancer cells with oleanolic and ursolic acid derived hydroxamates. Bioorganic Med. Chem. Lett. 2016, 26, 907–909. [Google Scholar] [CrossRef]
- Nedopekina, D.A.; Gubaidullin, R.R.; Odinokov, V.N.; Maximchik, P.V.; Zhivotovsky, B.; Bel’skii, Y.P.; Khazanov, V.A.; Manuylova, A.V.; Gogvadze, V.; Spivak, A.Y. Mitochondria-targeted betulinic and ursolic acid derivatives: Synthesis and anticancer activity†. Medchemcomm 2017, 8, 1934–1945. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.H.; Zhang, Z.H.; Li, M.Y.; Wei, Z.Y.; Jin, X.J.; Piao, H.R. Synthesis and evaluation of the HIF-1α inhibitory activities of novel ursolic acid tetrazole derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 1440–1445. [Google Scholar] [CrossRef]
- Kahnt, M.; Fischer, L.; Al-harrasi, A.; Csuk, R. Ethylenediamine Derived Carboxamides of Betulinic and Ursolic Acid as Potential Cytotoxic Agents. Molecules 2018, 23, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Lei, L.; Liu, Z.; Wang, H.; Meng, Q. Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules 2019, 24, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.Q.; Xu, C.D.; Yu, T.T.; Li, W.; Li, Q.W.; Li, X.X. Synthesis and antitumor activity evaluation of ursolic acid derivatives. J. Asian Nat. Prod. Res. 2020, 22, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, R.K.; Nee Heller, L.F.; Kluge, R.; Strohl, D.; Al-Harrasi, A.; Csuk, R. Chemistry Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans. Eur. J. Med. Chem. 2018, 155, 869–879. [Google Scholar] [CrossRef]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-harrasi, A.; Csuk, R. Synthesis and Cytotoxicity Evaluation of DOTA-Conjugates of Ursolic Acid. Molecules 2019, 24, 2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Zhang, L.; Liu, D.; Liu, L.; Zhang, Y. Synthesis and antitumor activity evaluation of novel ursolic acid derivatives. J. Asian Nat. Prod. Res. 2016, 18, 280–288. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF- κ B involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Z.H.; Zhang, L.H.; Jin, X.J.; Ma, J.; Piao, H.R. Design, synthesis, and screening of novel ursolic acid derivatives as potential anti-cancer agents that target the HIF-1α pathway. Bioorganic Med. Chem. Lett. 2019, 29, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Jin, X.; Li, D.; Wang, S.; Tao, X.; Chen, H. Design, Synthesis and in vitro anticancer activity of novel quinoline and oxadiazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2017, 27, 4128–4132. [Google Scholar] [CrossRef]
- Mendes, V.I.S.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Borková, L.; Frydrych, I.; Jakubcová, N.; Adámek, R.; Lišková, B.; Gurská, S.; Medvedíková, M.; Hajdúch, M.; Urban, M. Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. Eur. J. Med. Chem. 2020, 185, 111806. [Google Scholar]
- Fan, H.; Geng, L.; Yang, F.; Dong, X.; He, D.; Zhang, Y. Ursolic acid derivative induces apoptosis in glioma cells through down-regulation of cAMP. Eur. J. Med. Chem. 2019, 176, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.M.; Karpoormath, R.; Thapliyal, N.; Rane, R.A.; Palkar, M.B.; Faya, A.M.; Patel, H.M.; Alwan, W.S.; Jain, K.; Hampannavar, G.A. Current perspective of natural alkaloid carbazole and its derivatives as antitumor agents. Anticancer Agents. Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chem. Agents) 2015, 15, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.; Miao, T.; Zhang, K. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, L.; Zhao, Y.; Fu, S.; Liu, D.; Yu, Z. Three new triterpenoids transformed from ursolic acid by Mucor spinosus AS3. 3450 and their cytotoxicity. Phytochem. Lett. 2019, 32, 33–37. [Google Scholar] [CrossRef]
- Zhang, T.; He, B.; Yuan, H.; Feng, G.; Chen, F.; Wu, A.; Zhang, L. Synthesis and Antitumor Evaluation in Vitro of NO-Donating Ursolic Acid-Benzylidene Derivatives. Chem. Biodivers. 2019, 16, e1900111. [Google Scholar] [CrossRef]
- Jin, X.Y.; Chen, H.; Li, D.D.; Li, A.L.; Wang, W.Y.; Gu, W. Design, synthesis, and anticancer evaluation of novel quinoline derivatives of ursolic acid with hydrazide, oxadiazole, and thiadiazole moieties as potent MEK inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 955–972. [Google Scholar] [CrossRef] [Green Version]












| Plant Species (Family) | Plant Parts Used | Bioactivities | Bibliography |
|---|---|---|---|
| Arctostaphylos uva-ursi (L.) Spreng (Ericaceae) | Leaves | Antitumor, antibacterial | [15,16] |
| Argania spinosa (L.) Skeels (Sapotaceae) | Fruits, leaves | Antibacterial, antifungal | [17,18] |
| Bouvardia ternifolia (Cav.) Schltdl. (Rubiaceae) | Aerial parts | Anti-Alzheimer | [19] |
| Bursera cuneata (Schldl.) Engl (Burseraceae) | Aerial parts (stems and leaves) | Anti-inflammatory, antihistaminic | [20] |
| Catharanthus roseus (L.) G. Don (Apocynaceae) | Leaves | Anticancer | [21] |
| Cornus mas (L.) (Cornaceae) | Fruits | Antitumor | [22] |
| Eriobotrya japonica (Thunb.) Lindl (Rosaceae) | Leaves | Anti-cancer, anti-osteoclastic, skin disorder anti-inflammatory, and anti-arthritic | [23,24,25,26,27] |
| Eucalyptus globulus (Labill.) (Myrtaceae) | Leaves, bark | Antioxidant, neuroprotective | [28,29] |
| Fragraefragrans (Roxb.) (Gentianaceae) | Leaves, fruits, bark | Antimycobacterial | [30] |
| Ilex aquifolium (L.) (Aquifoliaceae) | Leaves | Anticancer, antimalarial, antibacterial | [31] |
| Lamium album (L.) (Lamiaceae) | Flowers | Antioxidant and anti-inflammatory | [32] |
| Lantana Camara (L.) (Verbenaceae) | Leaves | Antifungal, antiproliferative, anti-diabetes, anxiolytic | [33,34,35] |
| Lepidozia chordulifera (Dumort.) (Porellaceae) | Leaves | Antibacterial | [36] |
| Ligustrum lucidum (Ait.) (Oleaceae) | Fruits | Coronary heart disease and diabetes | [37,38] |
| Malus domestica (sp.) (Rosaceae) | Fruits, leaves | Antioxidant | [39] |
| Malus pumila (Mill.) (Rosaceae) | fruits | Antitumor | [40] |
| Ocimum forskolei (Benth.) (Lamiaceae) | Aerial parts (leaves and stems) | Antiulcer | [41] |
| Ocimum sanctum (L.) (Lamiaceae) | leaves | Induced arthritis, antiproliferative, anti-stress | [42,43,44] |
| Panax ginseng (C.A. Mey.) Baill. (Araliaceae) | Roots and rhizomes | Anticancer, antiviral | [45] |
| Paulownia tomentosa (Thunb.) Steud. (Scrophulariaceae) | Leaves | Anticancer | [46] |
| Prunella vulgaris (L.) (Lamiaceae) | Aerial parts | Antiviral, antiestrogenic | [47,48] |
| Psidium guajava (L.) (Myrtaceae) | Leaves | Hypoglycaemic, antimicrobial | [49] |
| Rabdosia rubescens (Linn.) (Lamiaceae) | Anti-tumour | Antitumor | [50] |
| Rosmarinus officinalis (L.) (Lamiaceae) | Stems and leaves | Antidepressant | [51] |
| Sambucus australis (Cham. & Schltdl.) (Adoxaceae) | Aerial parts | Antibacterial and Antioxidant | [52] |
| Saurauja roxburghii (Wall.) (Dilleniaceae) | Leaves | Anticancer | [53] |
| Thymus vulgaris (L.) (Lamiaceae) | Aerial parts (stems and leaves) | Anticancer, cardiovascular, antihyperlipidemic, antioxidant, antifungal | [54,55,56] |
| Tribulus arabicus (Hosni.) (Zygophyllaceae) | Aerial parts | Antihyperuricemic, antioxidant | [57,58] |
| Paulownia tomentos (Thunb.) Steud. (Scrophulariaceae) | Fruits | Anticancer | [46] |
| Punica granatum (Linn.) (Punicaceae) | Flowers | Antioxidant, antidiabetic | [59,60] |
| Uncaria rhynchophylla (Gouteng.) (Rubiaceae) | Stems and hooks | Anticancer | [61,62] |
| Vitex negundo (L.) (Lamiaceae) | Leaves | Antibacterial, antifeedant against the larvae | [63] |
| Ziziphus jujuba (Mill.) (Rhamnaceae) | Leaves | Anticancer, anti-obesity, and antioxidant | [64] |
| Sr. No. | Pharmacological Activities | Bibliography |
|---|---|---|
| 1 | Antioxidant | [102,103,104] |
| 2 | Antibacterial | [52,67,105,106,107,108,109,110] |
| 3 | Antifungal | [111,112,113,114,115,116,117] |
| 4 | Anticancer | [13,118,119,120,121,122,123] |
| 5 | Antidiabetic | [34,124,125,126,127,128] |
| 6 | Anti-inflammatory | [129,130,131,132,133] |
| 7 | Antiviral | [134,135,136] |
 | |||||
|---|---|---|---|---|---|
| Compound | R | Biological Activity | Cell Lines Tested IC50 (µM) | Reference Molecules IC50 (µM) | Bibliography |
| 9a |  | Antiproliferative | MCF-7 (8.45 ± 0.26) Hela (8.37 ± 0.11) A549 (10.06 ± 1.39) | MCF-7 (Gefitinib) 17.83 ± 7.85 Hela (Gefitinib) 15.40 ± 4.65 A549 (Gefitinib) 11.02 ± 3.27 | [155] |
| 13b |  | Anticancer activity | HRE (36.9) | n.d | [156] |
| 14 |  | Anticancer activity | MGC-803 (4.99 ± 0.40) Bcap-37 (8.56 ± 0.44) | MGC-803(UA) 26.51 ± 1.1 Bcap-37(UA) 31.39 ± 0.85 | [157] |
 | ||||||
|---|---|---|---|---|---|---|
| Compound | R1 | R2 | Biological Activities | Cell Lines Tested(IC50µM) | Reference Molecules (IC50µM) | Bibliography |
| 15 |  |  | Cytotoxicity | MGC-803 (9.82 ± 0.29) HCT-116 (18.97 ± 0.53) T24 (19.60 ± 0.43) HepG2 (15.72 ± 0.84) A549 (20.79 ± 0.54) HL-7702 (˃100) | MGC-803(UA) 27.08 ± 0.29 HCT-116(UA) 38.78 ± 0.16 T24(UA) 29.29 ± 0.80 HepG2(UA) 30.21± 0.58 A549(UA) 35.79 ± 0.37 HL-7702(˃100) | [139] |
| 16 |  |  | Cytotoxicity | 518A2 (3.6 ± 0.1) A2780 (2.7 ± 0.1) A549 (3.9 ± 0.1) FaDu (6.4 ± 0.4) HT29 (3.5 ± 0.3) MCF-7 (3.3 ± 0.2) NIH 3T3 (2.5 ± 0.6) | 518A2 (UA) 14.7 ± 0.1 A2780 (UA) 11.7 ± 0.6 A549 (UA) 15.5 ± 1.3 FaDu (UA) 14.2 ± 2.0 HT29 (UA) 10.6 ± 0.3 MCF-7 (UA) 12.7 ± 0.1 NIH 3T3 (UA)18.7 ± 1.6 | [158] |
| 17 |  |  | Cytotoxicity | TET21N (0.81 ± 0.08) MCF-7(1.59 ± 0.11) | TET21N (˃10) MCF-7(˃25) | [159] |
| 18 |  |  | T24(6.01 ± 0.87) A549(5.22 ± 0.65) HepG2(6.82 ± 1.07) SKOV3(8.95± 1.26) | T24(UA)37.88 ± 1.12 A549(UA) HepG2(UA) SKOV3(UA) | [94] | |
| 19 |  |  | Anti-cancer | HRE(0.8 ± 0.2) | HRE (UA) > 100 | [160] |
| 20 |  |  | Cytotoxicity | 518A2(2.7 ± 0.10) A2780(2.3 ± 0.10) HT29(1.8 ± 0.10) MCF-7(2.0 ± 0.10) 8505C(4.1 ± 0.40) NIH3T3(2.6 ± 0.30) | 518A2(UA) 14.7 ± 0.1 A2780(UA) 11.7 ± 0.6 HT29(UA) 10.6 ± 0.7 MCF-7(UA) 12.7 ± 0.1 8505C(UA) 13.5 ± 1.5 NIH 3T3(UA) 18.7 ± 1.6 | [161] |
| 21 |  |  | 518A2(3.2 ± 0.10) A2780(2.4 ±0.10) HT29(1.8 ± 0.20) MCF-7(2.7 ± 0.30) 8505C(5.4 ± 0.40) NIH 3T3(2.2 ± 0.10) | 518A2(UA) 14.7 ± 0.1 A2780(UA) 11.7 ± 0.6 HT29(UA) 10.6 ± 0.7 MCF-7(UA) 12.7 ± 0.1 8505C(UA) 13.5 ± 1.5 NIH 3T3(UA) 18.7 ± 1.6 | ||
| 22 |  |  | 518A2(2.7 ± 0.10) A2780(2.6 ± 0.10) HT29(1.7 ± 0.10) MCF-7(1.7 ± 0.10) 8505C(3.2 ± 0.01) NIH 3T3 (1.3 ± 0.20) | 518A2(UA) 14.7 ± 0.1 A2780(UA) 11.7 ± 0.6 HT29(UA) 10.6 ± 0.7 MCF-7(UA) 12.7 ± 0.1 8505C(UA) 13.5 ± 1.5 NIH 3T3(UA) 18.7 ± 1.6 | ||
| 23 |  |  | Cytotoxicity | Hela (2.6 ± 1.1) MKN45(2.1 ± 0.3) | Hela (Cisplatin) 15.1 ± 0.9 MKN45(Cisplatin) 2.8 ± 0.1 | [162] |
| 24 |  |  | Cytotoxicity | BEL-7402 (4.49) SGC-7901(7.01) | BEL-7402 (UA) >50 SGC-7901(UA) >50 | [163] |
| 25 |  |  | Cytotoxicity | A375(0.51 ± 0.05) A2780(0.45 ± 0.03) HT29(0.50 ± 0.07) MCF7(0.39 ± 0.04) NiH3T3(0.40 ± 0.03) SW1736 (n.d) | A375(BA) A2780(BA) HT29(BA) MCF7(BA) NiH3T3(BA) SW1736(BA) | [164] |
| 26 |  |  | Cytotoxicity | A375(1.5 ± 0.4) A2780(1.9 ± 0.3) HT29 (5.7 ± 0.5) MCF-7(4.4 ± 0.7) FaDu (3.7 ± 0.6) NIH 3T3(4.6 ± 1.0) | A375(UA) n.d. A2780(UA) 11.7 ± 0.6 HT29 (UA) 10.6 ± 0.7 MCF-7(UA) 12.7 ± 0.1 FaDu (UA) n.d NIH 3T3(UA) 13.1 ± 1.1 | [165] |
| 27 |  |  | A375(2.0 ± 0.1) A2780(1.7 ± 0.1) HT29 (2.3 ± 0.3) MCF-7(1.8 ± 0.1) FaDu (2.0 ± 0.2) NIH 3T3(1.4 ± 0.1) | A375(UA) n.d. A2780(UA) 11.7 ± 0.6 HT29 (UA) 10.6 ± 0.7 MCF-7(UA) 12.7 ± 0.1 FaDu (UA) n.d NIH 3T3(UA) 13.1 ± 1.1 | ||
| 28 |  |  | cytotoxicity | HeLa(9.25) HepG2(21.2) BGC-823(8.06) | HeLa(Gefitinib) 17.1 HepG2(Gefitinib) 20.7 BGC-823 Gefitinib 19.3 | [166] |
| 29 |  |  | HeLa(13.8) HepG2(23.7) BGC-823(9.15) | |||
| Compound | Biological Activity | Cell Lines Tested (IC50µM) | Reference Molecules (IC50µM) | Bibliography |
|---|---|---|---|---|
| 30 | Cytotoxicity | HA22T/VGH(31.0 ± 1.5) HepG2(28.0 ± 2.0) Hep3B(32.5 ± 2.5) | HA22T/VGH(UA) ˃ 100 HepG2(UA) ˃ 100 Hep3B(UA) ˃ 100 | [167] |
| 31 | Cytotoxicity | HA22T/VGH(31.0 ±1.5) HepG2(28.0 ± 2.0) Hep3B(32.5 ± 2.5) | HA22T/VGH(UA) ˃100 HepG2(UA) ˃ 100 Hep3B(UA) ˃ 100 | [167] |
| 32 | Cytotoxicity | A549 (6.07 ± 0.91) H1975(10.64 ± 1.94) MCF-7(22.27± 3.51) BGC-823 (17.10 ± 1.04) t-HSC/Cl-6 (29.12 ± 3.71) | A549 (mitomycin C) 28.14 ± 3.41 H1975(mitomycin C) 34.51 ± 3.06 MCF-7(mitomycin C) 44.08 ± 4.01 BGC-823 (mitomycin C) 37.94 ± 2.88 t-HSC/Cl-6 (mitomycin C) n.d | [140] |
| Compound | Activity | Cell Lines Tested (IC50µM) | Reference Molecules (IC50µM) | Bibliography |
|---|---|---|---|---|
| 35b | Cytotoxicity | HRE (4.0) | HRE(UA) >100 | [168] |
| 38b | Cytotoxicity | MDA-MB-231 (0.61 ± 0.07) HeLa (0.36 ± 0.05) SMMC-7721(12.49± 0.08) QSG-7701( > 40) | MDA-MB-231(UA) > 40 HeLa (UA) >40 SMMC-7721(UA) >40 QSG-7701(UA) n.d | [169] |
| 43a | Cytotoxicity | H460(4.5 ± 0.4) H322(6.8 ± 1.5) H460(6.7 ± 0.5) | H460(UA) 14.8 ± 0.6 H322(UA) 15.3 ± 2.8 H460 LKB1+/+(UA) 21.1 ± 1.6 | [170] |
| 43b | H460(5.3 ± 0.3) H322(7.3 ± 1.0) H460 LKB1+/+(7.8 ± 1.1) | |||
| 43c | H460(2.6 ± 0.9) H322(3.3 ± 0.9) H460 LKB1+/+(4.4 ± 0.6) | |||
| 45 | Cytotoxicity | CCRF-CEM (3.6) CEM-DNR (21.8) HCT116 (28.4) HCT116 p53−/−(29.8) K562 (38.8) K562-TAX (25.1) A549 (27.6) U2OS (20.7) BJ (49.5) MRC-5(29.3) | CCRF-CEM14 (44) 10.4 CEM-DNR (44) 34.0 HCT116(44) 34.0 HCT116 p53−/−(44) 49.7 K562 (44) >50 K562-TAX (44) 35.5 A549 (44) >50 U2OS (44) >50 BJ (44) >50 MRC-5(44) >50 | [171] |
| 46 | CCRF-CEM (4.7) CEM-DNR (28.2) HCT116 (32.1) HCT116 p53−/−(32.3) K562 (34.7) K562-TAX (29.0) A549 (42.5) U2OS (33.1) BJ (˃ 50) MRC-5(39.8) | |||
| 50 | Cytotoxicity | SMMC-7721 (1.08 ± 0.22) HepG2 (1.26 ± 0.17) | SMMC-7721(Doxorubicin) 0.62 ± 0.16 HepG2 (Doxorubicin) 0.77 ± 0.12) | [174] |
| 51 | Cytotoxicity | Hela (1.06) K562 (28.7) KB(35.6) | Hela (UA) 14.2 K562 (UA) 52.7 KB 3(UA) 42.9 | [175] |
| 52 | Cytotoxicity | HepG-2(65.8 ± 6.3) MCF-7(> 100) HT-29(4.28 ± 3.5) A549(78.39 ± 5.6) | HepG-2 (UA) 44.35 ± 4.9 MCF-7 (UA) > 100 HT-29 (UA) > 100 A549 (UA) > 100 | [176] |
| 53 | MEK inhibitors | MDA-MB-231 (1.84 ± 0.13) HeLa (1.18 ± 0.03) SMMC-7721 (17.48 ± 0.10) QSG-7701(40.59 ± 2.89) | MDA-MB-231 (Etoposide) 5.26 ± 1.21 HeLa (Etoposide) 2.98 ± 0.42 SMMC-7721 (Etoposide) 3.48 ± 0.35 QSG-7701(Etoposide) 28.75 ± 3.28 | [177] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int. J. Mol. Sci. 2020, 21, 5920. https://doi.org/10.3390/ijms21165920
Khwaza V, Oyedeji OO, Aderibigbe BA. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. International Journal of Molecular Sciences. 2020; 21(16):5920. https://doi.org/10.3390/ijms21165920
Chicago/Turabian StyleKhwaza, Vuyolwethu, Opeoluwa O. Oyedeji, and Blessing A. Aderibigbe. 2020. "Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update" International Journal of Molecular Sciences 21, no. 16: 5920. https://doi.org/10.3390/ijms21165920
APA StyleKhwaza, V., Oyedeji, O. O., & Aderibigbe, B. A. (2020). Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. International Journal of Molecular Sciences, 21(16), 5920. https://doi.org/10.3390/ijms21165920