Effect of Gelation Temperature on the Molecular Structure and Physicochemical Properties of the Curdlan Matrix: Spectroscopic and Microscopic Analyses
Abstract
:1. Introduction
2. Results
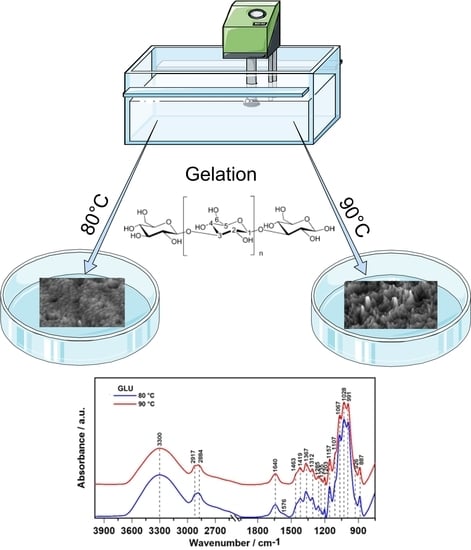
2.1. ATR FT-IR Spectroscopy
2.2. Raman Spectroscopy
2.3. X-ray Photoelectron Analysis
2.4. Contact Angle Measurements
2.5. Surface Topography Imaging
3. Discussion
4. Materials and Methods
4.1. Preparation of 1,3-β-d-glucan Samples
4.2. Spectroscopy
4.3. Raman Spectroscopy
4.4. X-ray Photoelectron Spectroscopy (XPS)
4.5. Contact Angle Measurements
4.6. Surface Topography Evaluation
4.6.1. Atomic Force Microscopy
4.6.2. Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATR FT-IR | Attenuated Total Reflection Fourier Transform Infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| AFM | Atomic Force Microscopy |
| SEM | Scanning Electron Microscopy |
| BSA | Bovine Serum Albumin |
| ATRA | All-trans Retinoic Acid |
| HepG2 | Hepatoma cell line |
References
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Boston, MA, USA, 2014; pp. 67–89. ISBN 9780123969835. [Google Scholar]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1→3)-β-d-glucans. Appl. Microbiol. Biotechnol. 2005, 68, 163–173. [Google Scholar] [CrossRef]
- Zhang, R.; Edgar, K.J. Properties, Chemistry, and Applications of the Bioactive Polysaccharide Curdlan. Biomacromolecules 2014, 15, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Masada, M.; Fujimori, K.; Maeda, I. Production of a Firm, Resilient Gel-forming Polysaccharide by a Mutant of Alcaligenes faecalis var. myxogenes 10 C3. Agric. Biol. Chem. 1966, 30, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Harada, T.; Misaki, A.; Saito, H. Curdlan: A bacterial gel-forming β-1, 3-glucan. Arch. Biochem. Biophys. 1968, 124, 292–298. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Da Silva, T.T.; Fenelon, V.C.; Koga, L.N.; de Souza Ferreira, S.B.; Bruschi, M.L.; Matioli, G. Description of recovery method used for curdlan produced by Agrobacterium sp. IFO 13140 and its relation to the morphology and physicochemical and technological properties of the polysaccharide. PLoS ONE 2017, 12, e0171469. [Google Scholar] [CrossRef] [PubMed]
- Mangolim, C.S.; da Silva, T.T.; Fenelon, V.C.; do Nascimento, A.; Sato, F.; Matioli, G. Use of FT-IR, FT-Raman and thermal analysis to evaluate the gel formation of curdlan produced by Agrobacterium sp. IFO 13140 and determination of its rheological properties with food applicability. Food Chem. 2017, 232, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Sletmoen, M.; Stokke, B.T. Structure-Function Relationships in Glycopolymers: Effects of Residue Sequences, Duplex, and Triplex Organization. Biopolymers 2013, 99, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Divyasri, D.; Gunasekar, V.; Benny, I.S.; Ponnusami, V. A review on industrial applications of curdlan. Int. J. ChemTech Res. 2014, 6, 3000–3003. [Google Scholar]
- Zhang, H.; Nishinari, K.; Williams, M.A.K.; Foster, T.J.; Norton, I.T. A molecular description of the gelation mechanism of curdlan. Int. J. Biol. Macromol. 2002, 30, 7–16. [Google Scholar] [CrossRef]
- Ortiz Martinez, C.; Pereira Ruiz, S.; Carvalho Fenelon, V.; Rodrigues de Morais, G.; Luciano Baesso, M.; Matioli, G. Characterization of curdlan produced by Agrobacterium sp. IFO 13140 cells immobilized in a loofa sponge matrix, and application of this biopolymer in the development of functional yogurt. J. Sci. Food Agric. 2016, 96, 2410–2417. [Google Scholar] [CrossRef]
- Gagnon, M.A.; Lafleur, M. From curdlan powder to the triple helix gel structure: An attenuated total reflection-Infrared study of the gelation process. Appl. Spectrosc. 2007, 61, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Cremer, L.; Lupu, A.R.; Badulescu, M.M.; Mocanu, G.; Mihai, D.; Calugaru, A.; Apetrei, N.S.; Moscovici, M.; Szegli, G. Assessment of two synthesized curdlan derivatives as possible antioxidants and/or modulators of human PMN cells respiratory burst. Rom. Biotechnol. Lett. 2010, 15, 5718–5728. [Google Scholar]
- Zhan, X.-B.; Lin, C.-C.; Zhang, H.-T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2012, 93, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Asai, M.; Kaneko, K.; Bae, A.-H.; Hasegawa, T.; Sakurai, K.; Shinkai, S. Inclusion of Cut and As-Grown Single-Walled Carbon Nanotubes in the Helical Superstructure of Schizophyllan and Curdlan (β-1,3-Glucans). J. Am. Chem. Soc. 2005, 127, 5875–5884. [Google Scholar] [CrossRef]
- Kanke, M.; Koda, K.; Koda, Y.; Katayama, H. Application of Curdlan to Controlled Drug Delivery. I. The Preparation and Evaluation of Theophylline-Containing Curdlan Tablets. Pharm. Res. 1992, 18, 1154–1158. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, I.D.; Kim, J.S.; Lee, J.H.; Lee, I.Y.; Lee, K.B. Curdlan gels as protein drug delivery vehicles. Biotechnol. Lett. 2000, 22, 1127–1130. [Google Scholar] [CrossRef]
- Saudagar, P.S.; Singhal, R.S. Curdlan as a support matrix for immobilization of enzyme. Carbohydr. Polym. 2004, 56, 483–488. [Google Scholar] [CrossRef]
- Hino, T.; Ishimoto, H.; Shimabayashi, S. Thermal gelation of aqueous curdlan suspension: Preparation of curdlan jelly. J. Pharm. Pharmacol. 2003, 55, 435–441. [Google Scholar] [CrossRef]
- Kanke, M.; Tanabe, E.; Katayama, H.; Koda, Y.; Yoshitomi, H. Application of Curdlan to Controlled Drug Delivery. III. Drug Release from Sustained Release Suppositories in Vitro. Biol. Pharm. Bull. 1995, 18, 1154–1158. [Google Scholar] [CrossRef] [Green Version]
- Soto, E.R.; Ostroff, G.R. Characterization of Multilayered Nanoparticles Encapsulated in Yeast Cell Wall Particles for DNA Delivery. Bioconjug. Chem. 2008, 19, 840–848. [Google Scholar] [CrossRef]
- Tesz, G.J.; Aouadi, M.; Prot, M.; Nicoloro, S.M.; Boutet, E.; Amano, S.U.; Goller, A.; Wang, M.; Guo, C.-A.; Salomon, W.E.; et al. Glucan particles for selective delivery of siRNA to phagocytic cells in mice. Biochem. J. 2011, 436, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, E.R.; Caras, A.C.; Kut, L.C.; Castle, M.K.; Ostroff, G.R. Glucan Particles for Macrophage Targeted Delivery of Nanoparticles. J. Drug Deliv. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, R.K.; Kang, K.W.; Choi, H.-K. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur. J. Pharm. Sci. 2009, 37, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Park, K.-H.; Kim, S.W.; Bae, Y.H. Self-assembled hydrogel nanoparticles from curdlan derivatives: Characterization, anti-cancer drug release and interaction with a hepatoma cell line (HepG2). J. Control. Release 2000, 69, 225–236. [Google Scholar] [CrossRef]
- Funami, T.; Funami, M.; Yada, H.; Nakao, Y. A rheological study on the effects of heating rate and dispersing method on the gelling characteristics of curdlan aqueous dispersions. Food Hydrocoll. 2000, 14, 509–518. [Google Scholar] [CrossRef]
- Upadhyay, T.K.; Fatima, N.; Sharma, D.; Saravanakumar, V.; Sharma, R. Preparation and characterization of beta-glucan particles containing a payload of nanoembedded rifabutin for enhanced targeted delivery to macrophages. EXCLI J. 2017, 16, 210–228. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef]
- Bollas, S.; Chrissopoulou, K.; Andrikopoulos, K.; Voyiatzis, G.; Anastasiadis, S. Polymer Conformation under Confinement. Polymers 2017, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Przekora, A.; Benko, A.; Blazewicz, M.; Ginalska, G. Hybrid chitosan/β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption. Biomed. Mater. 2016, 11, 045001. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Tamm, L.K.; Tatulian, S.A. Infrared spectroscopy of proteins and peptides in lipid bilayers. Q. Rev. Biophys. 1997, 30, 365–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, A.K.; Iconomidou, V.A.; Chryssikos, G.D.; Gionis, V.; Kumar, K.; Hamodrakas, S.J. Secondary structure of chorion proteins of the Lepidoptera Pericallia ricini and Ariadne merione by ATR FT-IR and micro-Raman spectroscopy. Int. J. Biol. Macromol. 2011, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Pelton, J.T.; McLean, L.R. Spectroscopic Methods for Analysis of Protein Secondary Structure. Anal. Biochem. 2000, 277, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Novák, M.; Synytsya, A.; Gomba, G.K.; Hejlová, A.; Blahovec, J.; Gedeon, O.; Slepička, P.; Maixner, J.; Čopíková, J. Properties of β-Glucan films from intact and disintegrated Saccharomyces cerevisiae cells. Chem. List. 2011, 105, 955–960. [Google Scholar]
- Zeise, I.; Heiner, Z.; Holz, S.; Joester, M.; Büttner, C.; Kneipp, J. Raman Imaging of Plant Cell Walls in Sections of Cucumis sativus. Plants 2018, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Agarwal, U.P. Analysis of Cellulose and Lignocellulose Materials by Raman Spectroscopy: A Review of the Current Status. Molecules 2019, 24, 1659. [Google Scholar] [CrossRef] [Green Version]
- James, B.J.; Smith, B.G. Surface structure and composition of fresh and bloomed chocolate analysed using X-ray photoelectron spectroscopy, cryo-scanning electron microscopy and environmental scanning electron microscopy. LWT-Food Sci. Technol. 2009, 42, 929–937. [Google Scholar] [CrossRef]
- Dhayal, M.; Ratner, D.M. XPS and SPR Analysis of Glycoarray Surface Density. Langmuir 2009, 25, 2181–2187. [Google Scholar] [CrossRef] [Green Version]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Mičušík, M.; Šimon, P. Beta-glucan complexes with selected nutraceuticals: Synthesis, characterization, and stability. J. Funct. Foods 2014, 8, 309–318. [Google Scholar] [CrossRef]
- Stone, B.A. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Elsevier: San Diego, CA, USA, 2009. [Google Scholar]
- Ercan, B.; Webster, T. Cell Response to Nanoscale Features and Its Implications in Tissue Regeneration: An Orthopedic Perspective. In Nanotechnology and Regenerative Engineering; CRC Press: Boca Raton, FL, USA, 2014; pp. 145–190. [Google Scholar]
- Le, X.; Poinern, G.E.J.; Ali, N.; Berry, C.M.; Fawcett, D. Engineering a Biocompatible Scaffold with Either Micrometre or Nanometre Scale Surface Topography for Promoting Protein Adsorption and Cellular Response. Int. J. Biomater. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kazarian, S.G.; Chan, K.L.A. Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim. Biophys. Acta Biomembr. 2006, 1758, 858–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.L.; Vardaki, M.Z.; Goldin, R.D.; Kazarian, S.G. Fourier transform infrared spectroscopic imaging of colon tissues: Evaluating the significance of amide I and C–H stretching bands in diagnostic applications with machine learning. Anal. Bioanal. Chem. 2019, 411, 6969–6981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szafraniec, E.; Kus, E.; Wislocka, A.; Kukla, B.; Sierka, E.; Untereiner, V.; Sockalingum, G.D.; Chlopicki, S.; Baranska, M. Raman spectroscopy-based insight into lipid droplets presence and contents in liver sinusoidal endothelial cells and hepatocytes. J. Biophotonics 2019, 12, e201800290. [Google Scholar] [CrossRef] [PubMed]
- Bartyzel, A.; Kaczor, A.A.; Głuchowska, H.; Pitucha, M.; Wróbel, T.M.; Matosiuk, D. Thermal and spectroscopic studies of 2,3,5-trisubstituted and 1,2,3,5-tetrasubstituted indoles as non-competitive antagonists of GluK1/GluK2 receptors. J. Therm. Anal. Calorim. 2018, 133, 935–944. [Google Scholar] [CrossRef] [Green Version]
- Prado, B.M.; Kim, S.; Özen, B.F.; Mauer, L.J. Differentiation of Carbohydrate Gums and Mixtures Using Fourier Transform Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 2005, 53, 2823–2829. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949–983. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, Y.; Li, Y.; Lv, M.; Li, P.; Xu, H.; Wang, L. Preparation and characterization of novel curdlan/chitosan blending membranes for antibacterial applications. Carbohydr. Polym. 2011, 84, 952–959. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Kanke, M.; Katayama, H.; Nakamura, M. Application of curdlan to controlled drug delivery. II. In vitro and in vivo drug release studies of theophylline-containing curdlan tablets. Biol. Pharm. Bull. 1995, 18, 1104–1108. [Google Scholar] [CrossRef] [Green Version]
- Yunus Basha, R.; Sampath, S.K.; Doble, M. Dual delivery of tuberculosis drugs via cyclodextrin conjugated curdlan nanoparticles to infected macrophages. Carbohydr. Polym. 2019, 218, 53–62. [Google Scholar] [CrossRef]
- Yunus Basha, R.; Sampath Kumar, T.S.; Selvaraj, R.; Doble, M. Silver Loaded Nanofibrous Curdlan Mat for Diabetic Wound Healing: An In Vitro and In Vivo Study. Macromol. Mater. Eng. 2018, 303, 1800234. [Google Scholar] [CrossRef]
- Emanuele, A.; Di Stefano, L.; Giacomazza, D.; Trapanese, M.; Palma-Vittorelli, M.B.; Palma, M.U. Time-Resolved study of network self-organization from a biopolymeric solution. Biopolymers 1991, 31, 859–864. [Google Scholar] [CrossRef]
- Mamaril, J.C.; Paner, E.T.; Palacpac, E.S. The production of gel-forming polysaccharides by Rhizobium sp. and curdlan by a mutant cultured in coconut water. Trans. Nat. Acad. Sci. Tech. 1988, 10, 339–349. [Google Scholar]
- Funami, T.; Yotsuzuka, F.; Yada, H.; Nakao, Y. Thermoirreversible Characteristics of Curdlan Gels in a Model Reduced Fat Pork Sausage. J. Food Sci. 2006, 63, 575–579. [Google Scholar] [CrossRef]









| Wavenumber/cm−1 | Assignment |
|---|---|
| 3300 | -CONH- |
| 2917 | CH3, CH2 |
| 2884 | C-H |
| 1640 | C=O |
| 1576 | COO−, CN |
| 1463 | CH2 |
| 1419 | CH2, CH3 |
| 1367 | CH, CH3 |
| 1312 | CH2 |
| 1285 | C-O (trans conformation) |
| 1235 | C-OH |
| 1203 | C-O, C-O-C |
| 1157 | C-O-C (ring) |
| 1107 | C-O |
| 1067 | C-O |
| 1028 | C-O |
| 991 | C-O, C-C |
| 926 | C-H |
| 887 | β-linked glycosidic bonds |
| Raman Shift/cm−1 | Assignment |
|---|---|
| 222 | C=C (bending) |
| 289 | C-C-C (def.) |
| 324 | C-C-C-C (out of plane bending) |
| 339 | C-CH3 (def.) |
| 358 | C-C-C (def.) |
| 395 | C-C(=O)C (def.) |
| 425 | HCC (out of plane bending) |
| 467 | C-C=O (in plane bending) |
| 527 | C-N=C (def.) |
| 570 | C-C-C (def.) |
| 607 | CH (out of plane bending) |
| 812 | CH (out-of-plane bending) |
| 891 | HCC, HCO, CH (def. out of plane, β-glucoside bond) |
| 938 | CH (def. out of plane) |
| 956 | CH (rings) (stretch.) |
| 998 | CC, COC (stretch.) |
| 1043 | CC, COH, CH (def.) |
| 1064 | CO (stretch. sym.) |
| 1092 | CC, CO (stretch.) |
| 1116 | COC (stretch.) |
| 1148 | COC (glycosidic bonds) |
| 1201 | CCH (def.) |
| 1227 | CH rings (stretch.) |
| 1254 | CCH (def.) |
| 1315 | CH, OH (def. in plane) |
| 1367 | CH, COH (def.) |
| 1411 | CO (stretch.) |
| 1423 | O-CH3, CH2 (def.) |
| 1461 | O-CH3, HCH, HOC, CH (def. asym.), CH2 (in plane bending) |
| Binding Energy/eV High Resolution Spectra | Chemical Composition | |||||
|---|---|---|---|---|---|---|
| GLU 80 °C | C1s | O1s | N1s | C:O At. % ratio (C:N) | species | O—32.6 N—2.7 Si—1.1 C—63.5 S—0.2 |
| 284.6 286.3 287.8 289.0 282.4 | 533.0 531.3 535.4 | 400.1 401.8 398.1 | 1.95 (23.51) | C-C/C-H C-OH, C-N O-C-O O=C-OH Si-C-O H2O C-NHx N3− | ||
| GLU 90 °C | C1s | O1s | N1s | C:O At. % ratio (C:N) | species | O—30.9 N—2.8 Si—0.3 C—65.3 S—0.8 |
| 284.6 286.2 287.8 289.1 282.7 | 532.8 531.3 535.8 | 399.9 402.0 398.1 | 2.11 (23.55) | C-C/C-H C-OH, C-N O-C-O O=C-OH Si-C-O H2O C-NHx N3− | ||
| Gelation Temperature | Surface Free Energy [mN/m] ± SD | Dispersive Part [mN/m] ± SD | Polar Part [mN/m] ± SD |
|---|---|---|---|
| Glu 80 °C | 37.72 ± 1.67 | 34.87 ± 0.95 | 2.85 ± 0.72 |
| Glu 90 °C | 37.59 ± 1.64 | 35.01 ± 1.00 | 2.58 ± 0.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Pieta, I.S.; Nowakowski, R.; Pisarek, M.; Przekora, A. Effect of Gelation Temperature on the Molecular Structure and Physicochemical Properties of the Curdlan Matrix: Spectroscopic and Microscopic Analyses. Int. J. Mol. Sci. 2020, 21, 6154. https://doi.org/10.3390/ijms21176154
Gieroba B, Sroka-Bartnicka A, Kazimierczak P, Kalisz G, Pieta IS, Nowakowski R, Pisarek M, Przekora A. Effect of Gelation Temperature on the Molecular Structure and Physicochemical Properties of the Curdlan Matrix: Spectroscopic and Microscopic Analyses. International Journal of Molecular Sciences. 2020; 21(17):6154. https://doi.org/10.3390/ijms21176154
Chicago/Turabian StyleGieroba, Barbara, Anna Sroka-Bartnicka, Paulina Kazimierczak, Grzegorz Kalisz, Izabela S. Pieta, Robert Nowakowski, Marcin Pisarek, and Agata Przekora. 2020. "Effect of Gelation Temperature on the Molecular Structure and Physicochemical Properties of the Curdlan Matrix: Spectroscopic and Microscopic Analyses" International Journal of Molecular Sciences 21, no. 17: 6154. https://doi.org/10.3390/ijms21176154
APA StyleGieroba, B., Sroka-Bartnicka, A., Kazimierczak, P., Kalisz, G., Pieta, I. S., Nowakowski, R., Pisarek, M., & Przekora, A. (2020). Effect of Gelation Temperature on the Molecular Structure and Physicochemical Properties of the Curdlan Matrix: Spectroscopic and Microscopic Analyses. International Journal of Molecular Sciences, 21(17), 6154. https://doi.org/10.3390/ijms21176154