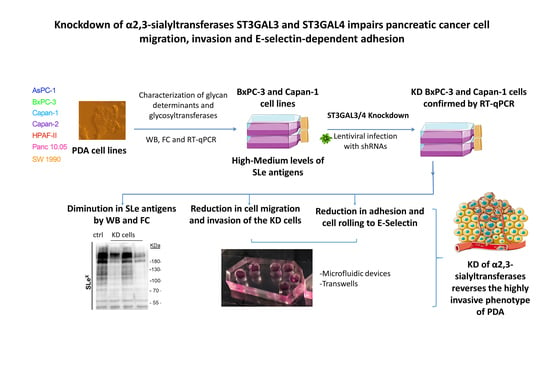
Knockdown of α2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion
Abstract
:1. Introduction
2. Results
2.1. Expression of Sialylated Glycan Determinants, α2,3-Sialyltransferases and α1,3/4-Fucosyltransferases in a Panel of PDA Cells
2.2. Stable Silencing of ST3GAL4 and ST3GAL3 in BxPC-3 and Capan-1 Cells
2.3. Downregulation of ST3GAL4 and ST3GAL3 in BxPC-3 and Capan-1 Cells Reduces sLex Expression
2.4. ST3GAL4 and ST3GAL3 Knockdown in BxPC-3 and Capan-1 Impaired Pancreatic Cancer Cell Migration
2.5. ST3GAL4 and ST3GAL3 Knockdown Reduced Cell Invasion In Vitro
2.6. Reduced Levels of sLex in ST3GAL4 and ST3GAL3 Knockdown Cells Led to Decreased Binding to E-Selectin
3. Discussion
3.1. Diversity in Sialyl-Lewis Antigens’ and their Corresponding Glycogenes’ Expression in PDA Cells
3.2. ST3GAL4 and ST3GAL3 Knockdown Effects on sLex/sLea Cell Levels
3.3. ST3GAL4 and ST3GAL3 Silencing Effects on Migration and Invasion Capabilities of the Tumor Cells
3.4. ST3GAL4 and ST3GAL3 Silencing Effects on E-selectin Binding of Tumor Cells
4. Materials and Methods
4.1. Cell Lines
4.2. Conditioned Media, Protein Lysates and Western Blot Analysis
4.3. Lentiviral Generation, Viral Transduction and Silencing by Short Hairpin RNA (shRNA)
4.4. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
4.5. Flow Cytometry Analysis
4.6. E-selectin Adhesion Assays
4.7. Transwell Migration Assay
4.8. Polydimethylsiloxane (PDMS)-Based Microchannel Migration Assay
4.9. Transwell Invasion Assay
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| KD | knockdown |
| mAb | monoclonal antibody |
| PDA | pancreatic ductal adenocarcinoma |
| SA | sialic acid |
| SC | scramble |
| sLe | sialyl-Lewis |
| sLex | sialyl-Lewis x |
| sLea | sialyl-Lewis a |
| ST | sialyltransferase |
| SD | standard deviation |
| SEM | standard error of the mean |
| WB | western blot |
References
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Bosch, N.; Guerrero, P.E.; Moreno, M.; José, A.; Iglesias, M.; Munné-Collado, J.; Anta, H.; Gibert, J.; Orozco, C.A.; Vinaixa, J.; et al. The pancreatic niche inhibits the effectiveness of sunitinib treatment of pancreatic cancer. Oncotarget 2016, 7, 48265–48279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Gomes, C.; Osório, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 Leads to SLex Expression and Induces c-Met Activation and an Invasive Phenotype in Gastric Carcinoma Cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef]
- Yoshihama, N.; Yamaguchi, K.; Chigita, S.; Mine, M.; Abe, M.; Ishii, K.; Kobayashi, Y.; Akimoto, N.; Mori, Y.; Sugiura, T. A novel function of CD82/KAI1 in sialyl Lewis antigen-mediated adhesion of cancer cells: Evidence for an anti-metastasis effect by down-regulation of sialyl Lewis antigens. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Garay, M.; Arteta, B.; Pagès, L.; De Llorens, R.; De Bolòs, C.; Vidal-Vanaclocha, F.; Peracaula, R. α2,3-Sialyltransferase ST3Gal III Modulates Pancreatic Cancer Cell Motility and Adhesion In Vitro and Enhances Its Metastatic Potential In Vivo. PLoS ONE 2010, 5, e12524. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Garay, M.; Arteta, B.; Llop, E.; Cobler, L.; Pagès, L.; Ortiz, R.; Ferri, M.J.; De Bolós, C.; Figueras, J.; De Llorens, R.; et al. α2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell Biol. 2013, 45, 1748–1757. [Google Scholar] [CrossRef]
- Fukuoka, K.; Narita, N.; Saijo, N. Increased expression of sialyl Lewis(x) antigen is associated with distant metastasis in lung cancer patients: Immunohistochemical study on bronchofiberscopic biopsy specimens. Lung Cancer 1998, 20, 109–116. [Google Scholar] [CrossRef]
- Julien, S.; Ivetic, A.; Grigoriadis, A.; QiZe, D.; Burford, B.; Sproviero, D.; Picco, G.; Gillett, C.; Papp, S.L.; Schaffer, L.; et al. Selectin ligand sialyl-lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011, 71, 7683–7693. [Google Scholar] [CrossRef] [PubMed]
- Kannagi, R.; Izawa, M.; Koike, T.; Miyazaki, K.; Kimura, N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004, 95, 377–384. [Google Scholar] [CrossRef]
- Tatsumi, M.; Watanabe, A.; Sawada, H.; Yamada, Y.; Shino, Y.; Nakano, H. Immunohistochemical expression of the sialyl Lewis x antigen on gastric cancer cells correlates with the presence of liver metastasis. Clin. Exp. Metastasis 1998, 16, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, P.; Mann, B.; Mansmann, U.; Lövin, N.; Foss, H.D.; Berger, G.; Scherübl, H.; Riecken, E.O.; Buhr, H.J.; Hanski, C. Expression of sialyl-Le(x) antigen defined by MAb AM-3 is an independent prognostic marker in colorectal carcinoma patients. Int. J. Cancer 2000, 88, 281–286. [Google Scholar] [CrossRef]
- Thomas, S.N.; Zhu, F.; Schnaar, R.L.; Alves, C.S.; Konstantopoulos, K. Carcinoembryonic Antigen and CD44 Variant Isoforms Cooperate to Mediate Colon Carcinoma Cell Adhesion to E- and L-selectin in Shear Flow. J. Biol. Chem. 2008, 283, 15647–15655. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Oda, T.; Hasebe, T.; Sasaki, S.; Kinoshita, T.; Konishi, M.; Ueda, T.; Nakahashi, C.; Ochiai, T.; Ochiai, A. Overexpression of Sialyl Lewis x Antigen Is Associated with Formation of Extratumoral Venous Invasion and Predicts Postoperative Development of Massive Hepatic Metastasis in Cases with Pancreatic Ductal Adenocarcinoma. Pathobiology 2001, 69, 127–135. [Google Scholar] [CrossRef]
- Chen, S.; Dallas, M.R.; Balzer, E.M.; Konstantopoulos, K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J. 2012, 26, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Bhide, G.P.; Colley, K.J. Sialylation of N-glycans: Mechanism, cellular compartmentalization and function. Histochem. Cell Biol. 2017, 147, 149–174. [Google Scholar] [CrossRef]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef]
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Petretti, T.; Schulze, B.; Schlag, P.; Kemmner, W. Altered mRNA expression of glycosyltransferases in human gastric carcinomas. Biochim. Biophys. Acta Gen. Subj. 1999, 1428, 209–218. [Google Scholar] [CrossRef]
- Gretschel, S.; Haensch, W.; Schlag, P.M.; Kemmner, W. Clinical Relevance of Sialyltransferases ST6GAL-I and ST3GAL-III in Gastric Cancer. Oncology 2003, 65, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Hebbar, M.; Krzewinski-Recchi, M.-A.; Hornez, L.; Verdière, A.; Harduin-Lepers, A.; Bonneterre, J.; Delannoy, P.; Peyrat, J.-P. Prognostic value of Tumoral Sialyltransferase Expression and Circulating E-Selectin Concentrations in Node-Negative Breast Cancer Patients. Int. J. Biol. Markers 2003, 18, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Recchi, M.A.; Hebbar, M.; Hornez, L.; Harduin-Lepers, A.; Peyrat, J.P.; Delannoy, P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998, 58, 4066–4070. [Google Scholar]
- Cui, H.-X.; Wang, H.; Wang, Y.; Song, J.; Tian, H.; Xia, C.; Shen, Y. ST3Gal III modulates breast cancer cell adhesion and invasion by altering the expression of invasion-related molecules. Oncol. Rep. 2016, 36, 3317–3324. [Google Scholar] [CrossRef] [Green Version]
- Wen, K.C.; Sung, P.L.; Hsieh, S.L.; Chou, Y.T.; Lee, O.K.S.; Wu, C.W.; Wang, P.H. α2,3-sialyltransferase type I regulates migration and peritoneal dissemination of ovarian cancer cells. Oncotarget 2017, 8, 29013–29027. [Google Scholar] [CrossRef]
- Wang, P.H.; Li, Y.F.; Juang, C.M.; Lee, Y.R.; Chao, H.T.; Ng, H.T.; Tsai, Y.C.; Yuan, C.C. Expression of sialyltransferase family members in cervix squamous cell carcinoma correlates with lymph node metastasis. Gynecol. Oncol. 2002, 86, 45–52. [Google Scholar] [CrossRef]
- Schneider, F.; Kemmner, W.; Haensch, W.; Franke, G.; Gretschel, S.; Karsten, U.; Schlag, P.M. Overexpression of sialyltransferase CMP-sialic acid: Galβ1,3GalNAc-R α6-sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 2001, 61, 4605–4611. [Google Scholar]
- Petretti, T.; Kemmner, W.; Schulze, B.; Schlag, P.M. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut 2000, 46, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Satomura, Y.; Sawabu, N.; Takemori, Y.; Ohta, H.; Watanabe, H.; Okai, T.; Watanabe, K.; Matsuno, H.; Konishi, F. Expression of Various Sialylated Carbohydrate Antigens in Malignant and Nonmalignant Pancreatic Tissues. Pancreas 1991, 6, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Balmaña, M.; Duran, A.; Gomes, C.; Llop, E.; López-Martos, R.; Ortiz, M.R.; Barrabés, S.; Reis, C.A.; Peracaula, R. Analysis of sialyl-Lewis x on MUC5AC and MUC1 mucins in pancreatic cancer tissues. Int. J. Biol. Macromol. 2018, 112, 33–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peracaula, R.; Tabarés, G.; López-Ferrer, A.; Brossmer, R.; De Bolós, C.; De Llorens, R. Role of sialyltransferases involved in the biosynthesis of Lewis antigens in human pancreatic tumour cells. Glycoconj. J. 2005, 22, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bassagañas, S.; Carvalho, S.; Dias, A.M.; Pérez-Garay, M.; Ortiz, M.R.; Figueras, J.; Reis, C.A.; Pinho, S.S.; Peracaula, R. Pancreatic Cancer Cell Glycosylation Regulates Cell Adhesion and Invasion through the Modulation of α2β1 Integrin and E-Cadherin Function. PLoS ONE 2014, 9, e98595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassagañas, S.; Pérez-Garay, M.; Peracaula, R. Cell Surface Sialic Acid Modulates Extracellular Matrix Adhesion and Migration in Pancreatic Adenocarcinoma Cells. Pancreas 2014, 43, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.; Dykstra, B.; Lee, J.; Ashline, D.J.; Reinhold, V.N.; Rossi, D.J.; Sackstein, R. Distinct human (1,3)-fucosyltransferases drive Lewis-X/sialyl Lewis-X assembly in human cells. J. Biol. Chem. 2018, 293, 7300–7314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedl, P.; Alexander, S. Cancer Invasion and the Microenvironment: Plasticity and Reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [Green Version]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Weigelin, B.; Bakker, G.-J.; Friedl, P. Intravital third harmonic generation microscopy of collective melanoma cell invasion. IntraVital 2012, 1, 32–43. [Google Scholar] [CrossRef]
- Hung, W.-C.; Chen, S.-H.; Paul, C.D.; Stroka, K.M.; Lo, Y.-C.; Yang, J.T.; Konstantopoulos, K. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J. Cell Biol. 2013, 202, 807–824. [Google Scholar] [CrossRef] [Green Version]
- Balzer, E.M.; Tong, Z.; Paul, C.D.; Hung, W.; Stroka, K.M.; Boggs, A.E.; Martin, S.S.; Konstantopoulos, K. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J. 2012, 26, 4045–4056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.; Balzer, E.M.; Dallas, M.R.; Hung, W.-C.; Stebe, K.J.; Konstantopoulos, K. Chemotaxis of Cell Populations through Confined Spaces at Single-Cell Resolution. PLoS ONE 2012, 7, e29211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, D.J.; Li, Y.W.; Stebe, K.J.; Konstantopoulos, K. E-selectin-mediated rolling facilitates pancreatic cancer cell adhesion to hyaluronic acid. FASEB J. 2017, 31, 5078–5086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jun, L.; Yuanshu, W.; Yanying, X.; Zhongfa, X.; Jian, Y.; Fengling, W.; Xianjun, Q.; Kokudo, N.; Wei, T.; Weixia, Z.; et al. Altered mRNA expressions of sialyltransferases in human gastric cancer tissues. Med. Oncol. 2012, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Leon, F.; Thompson, C.M.; Nimmakayala, R.; Karmakar, S.; Nallasamy, P.; Chugh, S.; Prajapati, D.R.; Rachagani, S.; Kumar, S.; et al. Global analysis of human glycosyltransferases reveals novel targets for pancreatic cancer pathogenesis. Br. J. Cancer 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.; Belo, A.I.; Giovannetti, E.; Van Die, I.; Wuhrer, M. Profiling of different pancreatic cancer cells used as models for metastatic behaviour shows large variation in their N-glycosylation. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mondal, N.; Buffone, A.; Stolfa, G.; Antonopoulos, A.; Lau, J.T.Y.; Haslam, S.M.; Dell, A.; Neelamegham, S. ST3Gal-4 is the primary sialyltransferase regulating the synthesis of E-, P-, and L-selectin ligands on human myeloid leukocytes. Blood 2015, 125, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhães, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of α-2,3-sialyltransferases and α-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef]
- Gupta, R.; Matta, K.L.; Neelamegham, S. A systematic analysis of acceptor specificity and reaction kinetics of five human α(2,3)sialyltransferases: Product inhibition studies illustrate reaction mechanism for ST3Gal-I. Biochem. Biophys. Res. Commun. 2016, 469, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.; Luo, Z.; Wu, J.; Qiu, L.; Luo, M.; Ke, Q.; Dong, X. Enhanced expression of α2,3-linked sialic acids promotes gastric cancer cell metastasis and correlates with poor prognosis. Int. J. Oncol. 2017, 50, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Aubert, M.; Panicot, L.; Crotte, C.; Gibier, P.; Lombardo, D.; Sadoulet, M.O.; Mas, E. Restoration of alpha(1,2) fucosyltransferase activity decreases adhesive and metastatic properties of human pancreatic cancer cells. Cancer Res. 2000, 60, 1449–1456. [Google Scholar] [PubMed]
- Aubert, M.; Panicot-Dubois, L.; Crotte, C.; Sbarra, V.; Lombardo, D.; Sadoulet, M.-O.; Mas, E. Peritoneal colonization by human pancreatic cancer cells is inhibited by antisenseFUT3 sequence. Int. J. Cancer 2000, 88, 558–565. [Google Scholar] [CrossRef]
- St. Hill, C.A.; Krieser, K.; Farooqui, M. Neutrophil interactions with sialyl Lewis X on human nonsmall cell lung carcinoma cells regulate invasive behavior. Cancer 2011, 117, 4493–4505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, P.; Chachadi, V.; Lin, M.-F.; Singh, R.; Kannagi, R.; Cheng, P.-W. TNFα enhances the motility and invasiveness of prostatic cancer cells by stimulating the expression of selective glycosyl- and sulfotransferase genes involved in the synthesis of selectin ligands. Biochem. Biophys. Res. Commun. 2011, 409, 436–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, Y.; Lin, H.; Liu, Y.; Tan, Y.; Lin, J.; Gao, F.; Lin, S. Alpha2,3-sialyltransferase III knockdown sensitized ovarian cancer cells to cisplatin-induced apoptosis. Biochem. Biophys. Res. Commun. 2017, 482, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, X.; Chen, M.; Zheng, S.; Li, J.; Lin, S.; Wang, X. ST3Gal3 confers paclitaxel-mediated chemoresistance in ovarian cancer cells by attenuating caspase-8/3 signaling. Mol. Med. Rep. 2019, 20, 4499–4506. [Google Scholar] [CrossRef]
- Sakuma, K.; Aoki, M.; Kannagi, R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2012, 109, 7776–7781. [Google Scholar] [CrossRef] [Green Version]
- Bull, C.; Boltje, T.J.; Wassink, M.; De Graaf, A.M.A.; van Delft, F.L.; Den Brok, M.H.; Adema, G.J. Targeting Aberrant Sialylation in Cancer Cells Using a Fluorinated Sialic Acid Analog Impairs Adhesion, Migration, and In Vivo Tumor Growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [Green Version]
- Büll, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; Van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; Van Der Vlag, J.; Heise, T.; et al. Sialic acid blockade suppresses tumor growth by enhancing t-cell-mediated tumor immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef] [Green Version]
- Sarrats, A.; Saldova, R.; Pla, E.; Fort, E.; Harvey, D.J.; Struwe, W.B.; De Llorens, R.; Rudd, P.M.; Peracaula, R. Glycosylation of liver acute-phase proteins in pancreatic cancer and chronic pancreatitis. PROTEOMICS Clin. Appl. 2010, 4, 432–448. [Google Scholar] [CrossRef]
- Martínez-Bosch, N.; Fernández-Barrena, M.G.; Moreno, M.; Ortiz-Zapater, E.; Munné-Collado, J.; Iglesias, M.; André, S.; Gabius, H.J.; Hwang, R.F.; Coise Poirier, F.; et al. Galectin-1 drives pancreatic carcinogenesis through stroma remodeling and hedgehog signaling activation. Cancer Res. 2014, 74, 3512–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejías-Luque, R.; López-Ferrer, A.; Garrido, M.; Fabra, À.; De Bolós, C. Changes in the invasive and metastatic capacities of HT-29/M3 cells induced by the expression of fucosyltransferase 1. Cancer Sci. 2007, 98, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.R.; Chen, S.H.; Streppel, M.M.; Sharma, S.; Maitra, A.; Konstantopoulos, K. Sialofucosylated podocalyxin is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells. Am. J. Physiol. Cell Physiol. 2012, 303, C616–C624. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Shea, D.J.; Mistriotis, P.; Tuntithavornwat, S.; Law, R.A.; Bieber, J.M.; Zheng, L.; Konstantopoulos, K. A Direct Podocalyxin–Dynamin-2 Interaction Regulates Cytoskeletal Dynamics to Promote Migration and Metastasis in Pancreatic Cancer Cells. Cancer Res. 2019, 79, 2878–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Cells | % change in sLex | Sig.1 | Sig.1 | % change in sLea | Sig.1 | % change in α2,6-SA | Sig.1 | Sig.1 |
|---|---|---|---|---|---|---|---|---|
| vs. SC cells 1 | vs. shST3GAL4_1 | vs. shST3GAL3_7 | vs. SC cells 1 | vs. shST3GAL3_7 | vs. SC cells 1 | vs. shST3GAL4_1 | vs. shST3GAL3_7 | |
| shST3GAL4_1 BxPC-3 | ↓68% (***) | - | ↑30%2 | ↑53% (***) | - | |||
| shST3GAL3_7 BxPC-3 | ↓33% (***) | *** | - | ↓2% (ns) | ↑21% (ns) | * | - | |
| shST3GAL3_9 BxPC-3 | ↓37% (***) | *** | ns | ↓34% (*) | * | ↑41% (**) | ns | ns |
| Cells | % change in sLex | Sig.1 | Sig.1 | Sig.1 | % change in sLea | Sig.1 | Sig.1 | Sig.1 | % change in α2,6-SA | Sig.1 | Sig.1 | Sig.1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vs. SC cells 1 | vs. shST3GAL4_1 | vs. shST3GAL4_4 | vs. shST3GAL3_7 | vs. SC cells 1 | vs. shST3GAL4_1 | vs. shST3GAL4_4 | vs. shST3GAL3_7 | vs. SC cells 1 | vs. shST3GAL4_1 | vs. shST3GAL4_4 | vs. shST3GAL3_7 | |
| shST3GAL4_1 Capan-1 | ↓64% (***) | - | ↓32% (*) | - | ↑35% (**) | - | ||||||
| shST3GAL4_4 Capan-1 | ↓63% (***) | ns | - | ↓45 (**) | ns | - | ↑24% (*) | ns | - | |||
| shST3GAL3_7 Capan-1 | ↓61% (***) | ns | ns | - | ↓43% (**) | ns | ns | - | ↑20% (ns) | ns | ns | - |
| shST3GAL3_9 Capan-1 | ↓73% (***) | ns | ns | ns | ↓52% (***) | ns | ns | ns | ↑12% (ns) | * | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, P.E.; Miró, L.; Wong, B.S.; Massaguer, A.; Martínez-Bosch, N.; Llorens, R.d.; Navarro, P.; Konstantopoulos, K.; Llop, E.; Peracaula, R. Knockdown of α2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion. Int. J. Mol. Sci. 2020, 21, 6239. https://doi.org/10.3390/ijms21176239
Guerrero PE, Miró L, Wong BS, Massaguer A, Martínez-Bosch N, Llorens Rd, Navarro P, Konstantopoulos K, Llop E, Peracaula R. Knockdown of α2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion. International Journal of Molecular Sciences. 2020; 21(17):6239. https://doi.org/10.3390/ijms21176239
Chicago/Turabian StyleGuerrero, Pedro Enrique, Laura Miró, Bin S. Wong, Anna Massaguer, Neus Martínez-Bosch, Rafael de Llorens, Pilar Navarro, Konstantinos Konstantopoulos, Esther Llop, and Rosa Peracaula. 2020. "Knockdown of α2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion" International Journal of Molecular Sciences 21, no. 17: 6239. https://doi.org/10.3390/ijms21176239
APA StyleGuerrero, P. E., Miró, L., Wong, B. S., Massaguer, A., Martínez-Bosch, N., Llorens, R. d., Navarro, P., Konstantopoulos, K., Llop, E., & Peracaula, R. (2020). Knockdown of α2,3-Sialyltransferases Impairs Pancreatic Cancer Cell Migration, Invasion and E-selectin-Dependent Adhesion. International Journal of Molecular Sciences, 21(17), 6239. https://doi.org/10.3390/ijms21176239