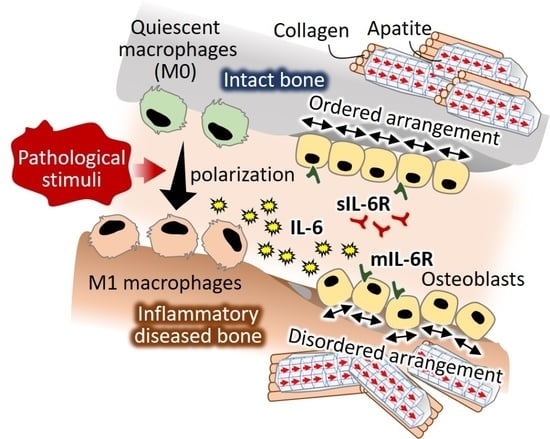
A Novel Role of Interleukin-6 as a Regulatory Factor of Inflammation-Associated Deterioration in Osteoblast Arrangement
Abstract
:1. Introduction
2. Results
2.1. Macrophage Polarization
2.2. The Effects of the Macrophage Subtype on Osteoblast Alignment
2.3. The Effects of IL-6 on Osteoblast Alignment
3. Discussion
4. Materials and Methods
4.1. Fabrication of the Oriented Collagen Substrates
4.2. Culture of Macrophages
4.3. Osteoblast Isolation and Culture
4.4. Alizarin Red S Staining
4.5. Anisotropic Co-Culture System
4.6. Fluorescence Imaging
4.7. Quantitative Analysis of Cell Alignment
4.8. Gene Expression and Protein Secretion Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakano, T.; Kaibara, K.; Ishimoto, T.; Tabata, Y.; Umakoshi, Y. Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 2012, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kaibara, K.; Tabata, Y.; Nagata, N.; Enomoto, S.; Marukawa, E.; Umakoshi, Y. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone 2002, 31, 479–487. [Google Scholar] [CrossRef]
- Liebi, M.; Georgiadis, M.; Menzel, A.; Schneider, P.; Kohlbrecher, J.; Bunk, O.; Guizar-Sicairos, M. Nanostructure surveys of macroscopic specimens by small-angle scattering tensor tomography. Nature 2015, 527, 349352. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Matsugaki, A.; Ishimoto, T.; Nakano, T. Evaluation of crystallographic orientation of biological apatite in vertebral cortical bone in ovariectomized cynomolgus monkeys treated with minodronic acid and alendronate. J. Bone Miner. Metab. 2016, 34, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kazama, J.J.; Yamato, H.; Matsugaki, A.; Nakano, T.; Fukagawa, M. Altered material properties are responsible for bone fragility in rats with chronic kidney injury. Bone 2015, 81, 247–254. [Google Scholar] [CrossRef]
- Wang, J.; Ishimoto, T.; Nakano, T. Unloading-Induced Degradation of the Anisotropic Arrangement of Collagen/Apatite in Rat Femurs. Calcif. Tissue Int. 2017, 100, 87–94. [Google Scholar] [CrossRef]
- Noyama, Y.; Nakano, T.; Ishimoto, T.; Sakai, T.; Yoshikawa, H. Design and optimization of the oriented groove on the hip implant surface to promote bone microstructure integrity. Bone 2013, 52, 659–667. [Google Scholar] [CrossRef]
- Sekita, A.; Matsugaki, A.; Nakano, T. Disruption of collagen/apatite alignment impairs bone mechanical function in osteoblastic metastasis induced by prostate cancer. Bone 2017, 97, 83–93. [Google Scholar] [CrossRef]
- Sekita, A.; Matsugaki, A.; Ishimoto, T.; Nakano, T. Synchronous disruption of anisotropic arrangement of the osteocyte network and collagen/apatite in melanoma bone metastasis. J. Struct. Biol. 2017, 197, 260–270. [Google Scholar] [CrossRef]
- Ishimoto, T.; Sato, B.; Lee, J.-W.; Nakano, T. Co-deteriorations of anisotropic extracellular matrix arrangement and intrinsic mechanical property in c-src deficient osteopetrotic mouse femur. Bone 2017, 103, 216–223. [Google Scholar] [CrossRef]
- Kimura, Y.; Matsugaki, A.; Sekita, A.; Nakano, T. Alteration of osteoblast arrangement via direct attack by cancer cells: New insights into bone metastasis. Sci. Rep. 2017, 7, 44824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsugaki, A.; Harada, T.; Kimura, Y.; Sekita, A.; Nakano, T. Dynamic Collision Behavior Between Osteoblasts and Tumor Cells Regulates the Disordered Arrangement of Collagen Fiber/Apatite Crystals in Metastasized Bone. Int. J. Mol. Sci. 2018, 19, 3474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsugaki, A.; Matsuzaka, T.; Murakami, A.; Wang, P.; Nakano, T. 3D Printing of Anisotropic Bone-Mimetic Structure with Controlled Fluid Flow Stimuli for Osteocytes: Flow Orientation Determines the Elongation of Dendrites. Int. J. Bioprint. 2020, 6, 293. [Google Scholar] [CrossRef]
- Matsugaki, A.; Isobe, Y.; Saku, T.; Nakano, T. Quantitative regulation of bone-mimetic, oriented collagen/apatite matrix structure depends on the degree of osteoblast alignment on oriented collagen substrates. J. Biomed. Mater. Res. 2015, 103, 489–499. [Google Scholar] [CrossRef]
- Ozasa, R.; Matsugaki, A.; Isobe, Y.; Saku, T.; Yun, H.-S.; Nakano, T. Construction of human induced pluripotent stem cell-derived oriented bone matrix microstructure by using in vitro engineered anisotropic culture model. J. Biomed. Mater. Res. 2018, 106, 360–369. [Google Scholar] [CrossRef] [Green Version]
- Dussold, C.; Gerber, C.; White, S.; Wang, X.; Qi, L.; Francis, C.; Capella, M.; Courbon, G.; Wang, J.; Li, C.; et al. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019, 7, 12. [Google Scholar] [CrossRef]
- Ozasa, R.; Ishimoto, T.; Miyabe, S.; Hashimoto, J.; Hirao, M.; Yoshikawa, H. Osteoporosis Changes Collagen/Apatite Orientation and Young’s Modulus in Vertebral Cortical Bone of Rat. Calcif. Tissue Int. 2019, 104, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Briot, K.; Geusens, P.; Bultink, I.E.; Lems, W.F.; Roux, C. Inflammatory diseases and bone fragility. Osteoporos Int. 2017, 28, 3301–3314. [Google Scholar] [CrossRef]
- Li, G.; Chen, M.; Li, X.; Cesta, A.; Lau, A.; Thabane, L. Frailty and risk of osteoporotic fractures in patients with rheumatoid arthritis: Data from the Ontario Best Practices Research Initiative. Bone 2019, 127, 129–134. [Google Scholar] [CrossRef]
- Croes, M.; Öner, F.C.; van Neerven, D.; Sabir, E.; Kruyt, M.C.; Blokhuis, T.J.; Dhert, W.J.A.; Alblasa, J. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone 2016, 84, 262–270. [Google Scholar] [CrossRef]
- Madel, M.; Ibáñez, L.; Wakkach, A.; De Vries, T.J.; Charles, J. Immune Function and Diversity of Osteoclasts in Normal and Pathological Conditions. Front. Immunol 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Nakano, T.; Umakoshi, Y.; Yamamoto, M.; Tabata, Y. Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using recombinant bone morphogenetic protein-2. J. Bone Miner. Res. 2013, 28, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving concepts in bone-immune interactions in health and disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef]
- Lee, J.-W.; Nakano, T.; Toyosawa, S.; Tabata, Y.; Umakoshi, Y. Areal distribution of preferential alignment of biological apatite (BAp) crystallite on cross-section of center of femoral diaphysis in osteopetrotic (op/op) mouse. Mater. Trans. 2007, 48, 337–342. [Google Scholar] [CrossRef]
- Bah, A.; Vergne, I. Macrophage Autophagy and Bacterial Infections. Front. Immunol 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Review Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsugaki, A.; Aramoto, G.; Ninomiya, T.; Sawada, H.; Hata, S.; Nakano, T. Abnormal arrangement of a collagen/apatite extracellular matrix orthogonal to osteoblast alignment is constructed by a nanoscale periodic surface structure. Biomaterials 2015, 37, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Matsugaki, A.; Kawahara, K.; Ninomiya, T.; Sawada, H.; Nakano, T. Biomaterials Unique arrangement of bone matrix orthogonal to osteoblast alignment controlled by Tspan11-mediated focal adhesion assembly. Biomaterials 2019, 209, 103–110. [Google Scholar] [CrossRef]
- Davis, G.E. Affinity of integrins for damaged extracellular matrix: αvβ3 binds to denatured collagen type I through RGD sites. Biochem. Biophys. Res. Commun. 1992, 182, 1025–1031. [Google Scholar] [CrossRef]
- Li, Y.; Bäckesjö, C.-M.; Haldosén, L.-A.; Lindgren, U. IL-6 receptor expression and IL-6 effects change during osteoblast differentiation. Cytokine 2008, 43, 165–173. [Google Scholar] [CrossRef]
- Kaneshiro, S.; Ebina, K.; Shi, K.; Higuchi, C. IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J. Bone Miner. Metab. 2014, 32, 378–392. [Google Scholar] [CrossRef]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127. [Google Scholar] [CrossRef]
- Yamaoka, K. Janus kinase inhibitors for rheumatoid arthritis. Curr. Opin. Chem. Biol. 2016, 32, 29–33. [Google Scholar] [CrossRef]
- Wei, Z.; Jiang, W.; Wang, H.; Hali, L.; Tang, B.; Liu, B.; Jiang, H.; Sun, X. The IL-6/STAT3 pathway regulates adhesion molecules and cytoskeleton of endothelial cells in thromboangiitis obliterans. Cell Signal. 2018, 44, 118–126. [Google Scholar] [CrossRef]
- Dian, F.H.; Hua, B.; Wang, S.Y. IL-6 increases podocyte motility via MLC-mediated focal adhesion impairment and cytoskeleton disassembly. J. Cell Physiol. 2018, 233, 7173–7181. [Google Scholar]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsugaki, A.; Matsumoto, S.; Nakano, T. A Novel Role of Interleukin-6 as a Regulatory Factor of Inflammation-Associated Deterioration in Osteoblast Arrangement. Int. J. Mol. Sci. 2020, 21, 6659. https://doi.org/10.3390/ijms21186659
Matsugaki A, Matsumoto S, Nakano T. A Novel Role of Interleukin-6 as a Regulatory Factor of Inflammation-Associated Deterioration in Osteoblast Arrangement. International Journal of Molecular Sciences. 2020; 21(18):6659. https://doi.org/10.3390/ijms21186659
Chicago/Turabian StyleMatsugaki, Aira, Shun Matsumoto, and Takayoshi Nakano. 2020. "A Novel Role of Interleukin-6 as a Regulatory Factor of Inflammation-Associated Deterioration in Osteoblast Arrangement" International Journal of Molecular Sciences 21, no. 18: 6659. https://doi.org/10.3390/ijms21186659
APA StyleMatsugaki, A., Matsumoto, S., & Nakano, T. (2020). A Novel Role of Interleukin-6 as a Regulatory Factor of Inflammation-Associated Deterioration in Osteoblast Arrangement. International Journal of Molecular Sciences, 21(18), 6659. https://doi.org/10.3390/ijms21186659