On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH
Abstract
:1. Introduction
2. Results
2.1. Auto Reaction of Genipin
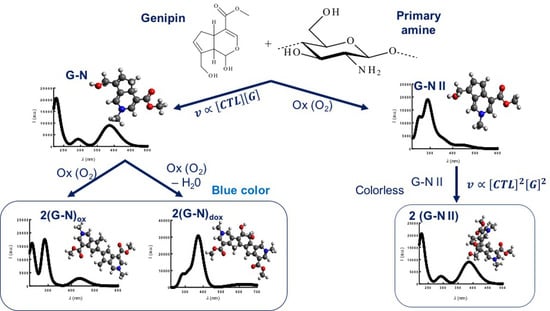
2.2. Binding of Genipin to Small Molecules Containing Amino Groups
2.3. Binding of Genipin to CTL at Neutral pH
2.4. Effect of Genipin and CTL Concentration on Binding Kinetics
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Lactose Modified Chitosan (CTL)
4.3. Synthesis of Lactose-Modified Chitosan at Reduced Molecular Weight (rCTL)
4.4. Binding of Genipin by Lactose-Modified Chitosan (CTL)
4.5. Intrinsic Viscosity Measurements
4.6. 1H-NMR
4.7. Circular Dichroism (CD)
4.8. UV-VIS Measurements
4.9. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sacco, P.; Cok, M.; Scognamiglio, F.; Pizzolitto, C.; Vecchies, F.; Marfoglia, A.; Marsich, E.; Donati, I. Glycosylated-Chitosan Derivatives: A Systematic Review. Molecules 2020, 25, 1534. [Google Scholar] [CrossRef] [Green Version]
- Esteban, C.; Donati, I.; Pantano, S.; Villegas, M.; Benegas, J.; Paoletti, S. Dissecting the conformational determinants of chitosan and chitlac oligomers. Biopolymers 2018, 109, e23221. [Google Scholar] [CrossRef]
- D’Amelio, N.; Esteban, C.; Coslovi, A.; Feruglio, L.; Uggeri, F.; Villegas, M.; Benegas, J.; Paoletti, S.; Donati, I. Insight into the Molecular Properties of Chitlac, a Chitosan Derivative for Tissue Engineering. J. Phys. Chem. B 2013, 117, 13578–13587. [Google Scholar] [CrossRef]
- Donati, I.; Borgogna, M.; Turello, E.; Cesàro, A.; Paoletti, S. Tuning Supramolecular Structuring at the Nanoscale Level: Nonstoichiometric Soluble Complexes in Dilute Mixed Solutions of Alginate and Lactose-Modified Chitosan (Chitlac). Biomacromolecules 2007, 8, 1471–1479. [Google Scholar] [CrossRef]
- Donati, I.; Haug, I.J.; Scarpa, T.; Borgogna, M.; Draget, K.I.; Skjåk-Bræk, A.G.; Paoletti, S. Synergistic Effects in Semidilute Mixed Solutions of Alginate and Lactose-Modified Chitosan (Chitlac). Biomacromolecules 2007, 8, 957–962. [Google Scholar] [CrossRef]
- Donati, I.; Stredanská, S.; Silvestrini, G.; Vetere, A.; Marcon, P.; Marsich, E.; Mozetic, P.; Gamini, A.; Paoletti, S.; Vittur, F. The aggregation of pig articular chondrocyte and synthesis of extracellular matrix by a lactose-modified chitosan. Biomaterials 2005, 26, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Borgogna, M.; Donati, I.; Mozetic, P.; Strand, B.L.; Salvador, S.G.; Vittur, F.; Paoletti, S. Alginate/lactose-modified chitosan hydrogels: A bioactive biomaterial for chondrocyte encapsulation. J. Biomed. Mater. Res. Part A 2007, 84, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Medelin, M.; Porrelli, D.; Aurand, E.R.; Scaini, D.; Travan, A.; Borgogna, M.A.; Cok, M.; Donati, I.; Marsich, E.; Scopa, C.; et al. Exploiting natural polysaccharides to enhance in vitro bio-constructs of primary neurons and progenitor cells. Acta Biomater. 2018, 73, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Foulc, M.-P.; Moritz, N.; Aro, H.T.; Paoletti, S. Polysaccharide-Coated Thermosets for Orthopedic Applications: From Material Characterization to In Vivo Tests. Biomacromolecules 2012, 13, 1564–1572. [Google Scholar] [CrossRef]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, P.; Furlani, F.; Paoletti, S.; Donati, I. pH-Assisted Gelation of Lactose-Modified Chitosan. Biomacromolecules 2019, 20, 3070–3075. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Sacco, P.; Scognamiglio, F.; Asaro, F.; Travan, A.; Borgogna, M.; Marsich, E.; Cok, M.; Paoletti, S.; Donati, I. Nucleation, reorganization and disassembly of an active network from lactose-modified chitosan mimicking biological matrices. Carbohydr. Polym. 2019, 208, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Furlani, F.; Sacco, P.; Cok, M.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Biomimetic, Multiresponsive, and Self-Healing Lactose-Modified Chitosan (CTL)-Based Gels Formed via Competitor-Assisted Mechanism. ACS Biomater. Sci. Eng. 2019, 5, 5539–5547. [Google Scholar] [CrossRef]
- Wahba, M.I. Enhancement of the mechanical properties of chitosan. J. Biomater. Sci. Polym. Ed. 2019, 31, 350–375. [Google Scholar] [CrossRef]
- Sung, H.; Huang, R.N.; Huang, L.L.H.; Tsai, C.-C.; Chiu, C.-T. Feasibility study of a natural crosslinking reagent for biological tissue fixation. J. Biomed. Mater. Res. 1998, 42, 560–567. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.-F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Touyama, R.; Inoue, K.; Takeda, Y.; Yatsuzuka, M.; Ikumoto, T.; Moritome, N.; Shingu, T.; Yokoi, T.; Inouye, H. Studies on the Blue Pigments Produced from Genipin and Methylamine. II. On the Formation Mechanisms of Brownish-Red Intermediates Leading to the Blue Pigment Formation. Chem. Pharm. Bull. 1994, 42, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Di Tommaso, S.; David, H.; Gomar, J.; Leroy, F.; Adamo, C. From iridoids to dyes: A theoretical study on genipin reactivity. RSC Adv. 2014, 4, 11029. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; El Mehtedi, M.; Bottegoni, C.; Gigante, A. Physical properties imparted by genipin to chitosan for tissue regeneration with human stem cells: A review. Int. J. Biol. Macromol. 2016, 93, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef]
- Heimbuck, A.M.; Priddy-Arrington, T.R.; Padgett, M.L.; Llamas, C.B.; Barnett, H.H.; Bunnell, B.A.; Caldorera-Moore, M.E. Development of Responsive Chitosan–Genipin Hydrogels for the Treatment of Wounds. ACS Appl. Bio Mater. 2019, 2, 2879–2888. [Google Scholar] [CrossRef]
- Mishra, A.H.; Mishra, D. Evidences of Biomimetic and Nonantibiotic Characteristics of the Zinc–Carboxymethyl Chitosan–Genipin Organometallic Complex and Its Biocompatibility Aspects. Biomacromolecules 2019, 21, 688–700. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.; Utturkar, G.; Haggard, W.; Yang, Y.; Ong, J.L.; Bumgardner, J.D. The effect of cross-linking of chitosan microspheres with genipin on protein release. Carbohydr. Polym. 2007, 68, 561–567. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.; Barralet, J.E.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Liang, H.-C.; Chang, Y.; Hsu, C.-K.; Lee, M.-H.; Sung, H. Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 2004, 25, 3541–3552. [Google Scholar] [CrossRef]
- Yang, M.-C.; Wang, S.-S.; Chou, N.-K.; Chi, N.-H.; Huang, Y.-Y.; Chang, Y.-L.; Shieh, M.-J.; Chung, T.-W. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin–polysaccharide cardiac patches in vitro. Biomaterials 2009, 30, 3757–3765. [Google Scholar] [CrossRef]
- Dimida, S.; Barca, A.; Cancelli, N.; De Benedictis, V.; Raucci, M.G.; Demitri, C. Effects of Genipin Concentration on Cross-Linked Chitosan Scaffolds for Bone Tissue Engineering: Structural Characterization and Evidence of Biocompatibility Features. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.-Y.; Li, Y.-T.; Wang, T.-P. In Vitro Response of Retinal Pigment Epithelial Cells Exposed to Chitosan Materials Prepared with Different Cross-Linkers. Int. J. Mol. Sci. 2010, 11, 5256–5272. [Google Scholar] [CrossRef] [Green Version]
- Dimida, S.; Demitri, C.; De Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Di Tommaso, S.; David, P.; Picolet, K.; Gabant, M.; David, H.; Morançais, J.-L.; Gomar, J.; Leroy, F.; Adamo, C. Structure of genipin in solution: A combined experimental and theoretical study. RSC Adv. 2013, 3, 13764. [Google Scholar] [CrossRef]
- Pirrung, M.C. Acceleration of Organic Reactions through Aqueous Solvent Effects. Chem. A Eur. J. 2006, 12, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, X.; Lu, Y. Surface modification of polyacrylonitrile-based carbon fiber and its interaction with imide. Appl. Surf. Sci. 2006, 253, 2695–2701. [Google Scholar] [CrossRef]
- Otto, S.; Engberts, J.B.F.N. Diels_Alder reactions in water. Pure Appl. Chem. 2000, 72, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Frederiksen, S.M.; Stermitz, F.R. Pyridine Monoterpene Alkaloid Formation from Iridoid Glycosides. A Novel PMTA Dimer from Geniposide. J. Nat. Prod. 1996, 59, 41–46. [Google Scholar] [CrossRef]
- Augé, S.; Schmit, P.-O.; Crutchfield, C.A.; Islam, M.T.; Harris, D.J.; Durand, E.; Clémancey, M.; Quoineaud, A.-A.; Lancelin, J.-M.; Prigent, Y.; et al. NMR Measure of Translational Diffusion and Fractal Dimension. Application to Molecular Mass Measurement. J. Phys. Chem. B 2009, 113, 1914–1918. [Google Scholar] [CrossRef]
- Viel, S.; Capitani, D.; Mannina, L.; Segre, A. Diffusion-Ordered NMR Spectroscopy: A Versatile Tool for the Molecular Weight Determination of Uncharged Polysaccharides. Biomacromolecules 2003, 4, 1843–1847. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Marsich, E.; Donati, I.; Paoletti, S. Highly monodisperse colloidal coacervates based on a bioactive lactose-modified chitosan: From synthesis to characterization. Carbohydr. Polym. 2017, 174, 360–368. [Google Scholar] [CrossRef]
- Christensen, B.E.; Vold, I.M.N.; Vårum, K.M. Chain stiffness and extension of chitosans and periodate oxidised chitosans studied by size-exclusion chromatography combined with light scattering and viscosity detectors. Carbohydr. Polym. 2008, 74, 559–565. [Google Scholar] [CrossRef]
- Sacco, P.; Cok, M.; Asaro, F.; Paoletti, S.; Donati, I. The role played by the molecular weight and acetylation degree in modulating the stiffness and elasticity of chitosan gels. Carbohydr. Polym. 2018, 196, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Tømmeraas, K.; Vårum, K.M.; Christensen, B.E.; Smidsrød, O. Preparation and characterisation of oligosaccharides produced by nitrous acid depolymerisation of chitosans. Carbohydr. Res. 2001, 333, 137–144. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; Marfoglia, A.; Cok, M.; Pizzolitto, C.; Marsich, E.; Donati, I. Temporary/Permanent Dual Cross-link Gels formed of a Bioactive Lactose-Modified Chitosan. Macromol. Biosci. 2020. [Google Scholar] [CrossRef]
- Antalek, B. Using pulsed gradient spin echo NMR for chemical mixture analysis: How to obtain optimum results. Concepts Magn. Reson. 2002, 14, 225–258. [Google Scholar] [CrossRef]
- Morris, K.F.; Johnson, C.S. Resolution of discrete and continuous molecular size distributions by means of diffusion-ordered 2D NMR spectroscopy. J. Am. Chem. Soc. 1993, 115, 4291–4299. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Chem. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Martynov, A.G.; Mack, J.; May, A.K.; Nyokong, T.; Gorbunova, Y.G.; Tsivadze, A.Y. Methodological Survey of Simplified TD-DFT Methods for Fast and Accurate Interpretation of UV–Vis–NIR Spectra of Phthalocyanines. ACS Omega 2019, 4, 7265–7284. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzolitto, C.; Cok, M.; Asaro, F.; Scognamiglio, F.; Marsich, E.; Lopez, F.; Donati, I.; Sacco, P. On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH. Int. J. Mol. Sci. 2020, 21, 6831. https://doi.org/10.3390/ijms21186831
Pizzolitto C, Cok M, Asaro F, Scognamiglio F, Marsich E, Lopez F, Donati I, Sacco P. On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH. International Journal of Molecular Sciences. 2020; 21(18):6831. https://doi.org/10.3390/ijms21186831
Chicago/Turabian StylePizzolitto, Chiara, Michela Cok, Fioretta Asaro, Francesca Scognamiglio, Eleonora Marsich, Francesco Lopez, Ivan Donati, and Pasquale Sacco. 2020. "On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH" International Journal of Molecular Sciences 21, no. 18: 6831. https://doi.org/10.3390/ijms21186831
APA StylePizzolitto, C., Cok, M., Asaro, F., Scognamiglio, F., Marsich, E., Lopez, F., Donati, I., & Sacco, P. (2020). On the Mechanism of Genipin Binding to Primary Amines in Lactose-Modified Chitosan at Neutral pH. International Journal of Molecular Sciences, 21(18), 6831. https://doi.org/10.3390/ijms21186831