Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery
Abstract
:1. Introduction
2. Methods
3. Results
4. Subjects in Included Studies
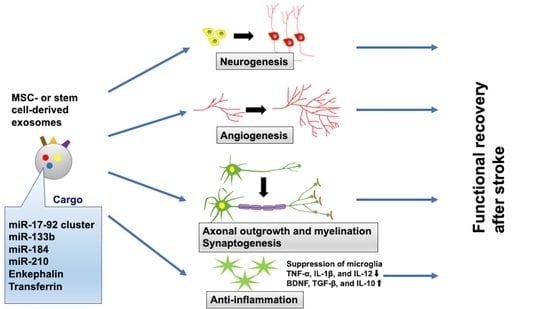
5. Therapeutic Effect of Exosomes for Stroke Recovery
5.1. Exosomes Derived from MSCs
5.2. Amplification of Specific Molecules in MSC-Generated Exosomes
6. Therapeutic Effect of Exosomes Compared with Cell Therapy
7. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.B.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Feigin, V.L.; Abajobir, A.A.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abera, S.F.; Abyu, G.Y.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; et al. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Executive summary: Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Badhiwala, J.H.; Nassiri, F.; Alhazzani, W.; Selim, M.H.; Farrokhyar, F.; Spears, J.; Kulkarni, A.V.; Singh, S.; Alqahtani, A.; Rochwerg, B.; et al. Endovascular thrombectomy for acute ischemic stroke: A meta-analysis. Jama 2015, 314, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Lees, K.R.; Bluhmki, E.; Von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A.; et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef]
- Mistry, E.A.; Mistry, A.M.; Nakawah, M.O.; Chitale, R.V.; James, R.F.; Volpi, J.J.; Fusco, M.R. Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients. Stroke 2017, 48, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Rabinstein, A. Symptomatic Intracranial Hemorrhage following Intravenous Thrombolysis for Acute Ischemic Stroke: A Critical Review of Case Definitions. Cerebrovasc. Dis. 2012, 34, 106–114. [Google Scholar] [CrossRef]
- Yaghi, S.; Willey, J.Z.; Cucchiara, B.; Goldstein, J.N.; Gonzales, N.R.; Khatri, P.; Kim, L.J.; Mayer, S.A.; Sheth, K.N.; Schwamm, L. Treatment and Outcome of Hemorrhagic Transformation After Intravenous Alteplase in Acute Ischemic Stroke: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e343–e361. [Google Scholar] [CrossRef]
- Kobayashi, S.; Fukuma, S.; Ikenoue, T.; Fukuhara, S.; Kobayashi, S.; Japan Stroke Data Bank. Effect of Edaravone on Neurological Symptoms in Real-World Patients with Acute Ischemic Stroke. Stroke 2019, 50, 1805–1811. [Google Scholar] [CrossRef]
- Langhorne, P.; Ramachandra, S. Stroke Unit Trialists’ Collaboration Organised inpatient (stroke unit) care for stroke: Network meta-analysis. Cochrane Database Syst. Rev. 2020, 4, CD000197. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Frutos, M.C.G.-D.; Laso-García, F.; Sánchez-Gonzalo, A.; Martínez-Arroyo, A.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. NogoA Neutralization Promotes Axonal Restoration After White Matter Injury In Subcortical Stroke. Sci. Rep. 2017, 7, 9431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.-S.; Dong, B.-C.; Li, M.-X.; Wang, X.-Y.; Cheng, X.; Wang, Y.; Xiao, T.; Jolkkonen, J.; Zhao, C.-S. Effects of CXCR7-neutralizing antibody on neurogenesis in the hippocampal dentate gyrus and cognitive function in the chronic phase of cerebral ischemia. Neural Regen. Res. 2020, 15, 1079–1085. [Google Scholar] [CrossRef]
- Hira, K.; Ueno, Y.; Tanaka, R.; Miyamoto, N.; Yamashiro, K.; Inaba, T.; Urabe, T.; Okano, H.; Hattori, N. Astrocyte-Derived Exosomes Treated With a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase. Stroke 2018, 49, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Chopp, M.; Zhang, L.; Buller, B.; Liu, Z.; Lehman, N.L.; Liu, X.S.; Zhang, Y.; Roberts, C.; Zhang, Z.G. Axonal outgrowth and dendritic plasticity in the cortical peri-infarct area after experimental stroke. Stroke 2012, 43, 2221–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwamori, H.; Higuchi, T.; Arai, K.; Sudo, R. Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Sci. Rep. 2017, 7, 17349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Mahmood, A.; Chopp, M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs (London Engl. 2000) 2010, 11, 298–308. [Google Scholar]
- Wu, Q.; Wang, Y.; Demaerschalk, B.M.; Ghimire, S.; Wellik, K.E.; Qu, W. Bone marrow stromal cell therapy for ischemic stroke: A meta-analysis of randomized control animal trials. Int. J. Stroke 2016, 12, 273–284. [Google Scholar] [CrossRef]
- Yan, T.; Venkat, P.; Chopp, M.; Zacharek, A.; Ning, R.; Roberts, C.; Zhang, Y.; Lu, M.; Chen, J. Neurorestorative Responses to Delayed Human Mesenchymal Stromal Cells Treatment of Stroke in Type 2 Diabetic Rats. Stroke 2016, 47, 2850–2858. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Wu, H.; Zhao, Y.; Guo, Y.; Chen, Y.; Dong, P.; Mu, Q.; Wang, X.; Chen, W. Bone marrow mesenchymal stromal cells alleviate brain white matter injury via the enhanced proliferation of oligodendrocyte progenitor cells in focal cerebral ischemic rats. Brain Res. 2017, 1680, 127–136. [Google Scholar] [CrossRef]
- Zhang, Y.; Ueno, Y.; Liu, X.S.; Buller, B.; Wang, X.; Chopp, M.; Zhang, Z.G. The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J. Neurosci. 2013, 33, 6885–6894. [Google Scholar] [CrossRef] [Green Version]
- Ueno, Y.; Koike, M.; Shimada, Y.; Shimura, H.; Hira, K.; Tanaka, R.; Uchiyama, Y.; Hattori, N.; Urabe, T. L-carnitine enhances axonal plasticity and improves white-matter lesions after chronic hypoperfusion in rat brain. J. Cereb. Blood Flow Metab. 2015, 35, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cheng, Q.; Hu, G.; Deng, T.; Wang, Q.; Zhou, J.; Su, X. Extracellular vesicles in mesenchymal stromal cells: A novel therapeutic strategy for stroke. Exp. Ther. Med. 2018, 15, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Hung, M.; Breakefield, X.O.; Leonard, J.N. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 2014, 55, 439–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzenbach, H.; Gahan, P.B. MicroRNA Shuttle from Cell-To-Cell by Exosomes and Its Impact in Cancer. Non-Coding RNA 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Gu, D.; Xing, X.; Cheng, Z.; Gong, D.; Zhang, G.; Zhu, Y. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am. J. Transl. Res. 2016, 8, 4289–4299. [Google Scholar] [PubMed]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.-H.; Lu, M.; Zhu, Y.-J. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; Van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [Google Scholar] [CrossRef]
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.-J.; Yu, X.-Y.; et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Wang, B.; Jia, H.; Zhang, B.; Wang, J.; Ji, C.; Zhu, X.; Yan, Y.; Yin, L.; Yu, J.; Qian, H.; et al. Pre-incubation with hucMSC-exosomes prevents cisplatin-induced nephrotoxicity by activating autophagy. Stem Cell Res. Ther. 2017, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.G.; Chopp, M. Exosomes in stroke pathogenesis and therapy. J. Clin. Investig. 2016, 126, 1190–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.G.; Buller, B.; Chopp, M. Exosomes—Beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019, 15, 193–203. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 6, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Janowska, J.; Gargas, J.; Ziemka-Nalecz, M.; Zalewska, T.; Sypecka, J. Oligodendrocyte Response to Pathophysiological Conditions Triggered by Episode of Perinatal Hypoxia-Ischemia: Role of IGF-1 Secretion by Glial Cells. Mol. Neurobiol. 2020, 57, 4250–4268. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Li, Y.; Cui, Y.; Yang, J.J.; Zhang, Z.G.; Chopp, M. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Promote Functional Recovery and Neurovascular Plasticity After Stroke in Rats. Br. J. Pharmacol. 2013, 33, 1711–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Gan, Y.; Xu, G.; Yin, G.; Liu, D. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem. Res. 2020, 45, 1180–1190. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Herz, J.; Görgens, A.; Schlechter, J.; Ludwig, A.-K.; Radtke, S.; De Miroschedji, K.; Horn, P.A.; Giebel, B.; Hermann, D.M. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. STEM CELLS Transl. Med. 2015, 4, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Ling, X.; Zhang, G.; Xia, Y.; Zhu, Q.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Yang, Y.; Wang, Y.; et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell. Mol. Med. 2020, 24, 640–654. [Google Scholar] [CrossRef] [Green Version]
- Nalamolu, K.R.; Venkatesh, I.; Mohandass, A.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Exosomes Treatment Mitigates Ischemic Brain Damage but Does Not Improve Post-Stroke Neurological Outcome. Cell. Physiol. Biochem. 2019, 52, 1280–1291. [Google Scholar] [CrossRef]
- Nalamolu, K.R.; Venkatesh, I.; Mohandass, A.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Kunamneni, A.; Veeravalli, K.K. Exosomes Secreted by the Cocultures of Normal and Oxygen–Glucose-Deprived Stem Cells Improve Post-stroke Outcome. NeuroMolecular Med. 2019, 21, 529–539. [Google Scholar] [CrossRef]
- Moon, G.J.; Sung, J.H.; Kim, N.H.; Kim, E.H.; Cho, Y.H.; Son, J.P.; Cha, J.M.; Bang, O.Y. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl. Stroke Res. 2018, 10, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Safakheil, M.; Safakheil, H. The Effect of Exosomes Derived from Bone Marrow Stem Cells in Combination with Rosuvastatin on Functional Recovery and Neuroprotection in Rats After Ischemic Stroke. J. Mol. Neurosci. 2020, 70, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Wang, F.; Li, Y.; Lu, Q.-E.; Cheung, W.L.; Zhang, Y.; Zhang, Z.G.; Chopp, M. Secondary Release of Exosomes from Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery after Stroke in Rats Treated with Exosomes Harvested from MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transpl. 2017, 26, 243–257. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Katakowski, M.; Wang, F.; Qian, J.-Y.; Liu, X.S.; Ali, M.M.; Buller, B.; Zhang, Z.G.; Chopp, M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke 2017, 48, 747–753. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, N.; Su, J.; Wang, X.; Li, X. Rapid Enkephalin Delivery Using Exosomes to Promote Neurons Recovery in Ischemic Stroke by Inhibiting Neuronal p53/Caspase-3. BioMed Res. Int. 2019, 2019, 4273290. [Google Scholar] [CrossRef]
- Geng, W.; Tang, H.; Luo, S.; Lv, Y.; Liang, D.; Kang, X.; Hong, W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019, 11, 780–792. [Google Scholar]
- Chen, K.-H.; Chen, C.-H.; Wallace, C.G.; Yuen, C.-M.; Kao, G.-S.; Chen, Y.-L.; Shao, P.-L.; Chen, Y.-L.; Chai, H.-T.; Lin, K.-C.; et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget 2016, 7, 74537–74556. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Wang, S.-Y.; Zeng, Q.-W.; Su, D.-S.; Li, W.; Wang, X.-R.; Zhao, Z. Astroglial reaction to delta opioid peptide [d-Ala2, d-Leu5] enkephalin confers neuroprotection against global ischemia in the adult rat hippocampus. Neuroscience 2011, 192, 81–90. [Google Scholar] [CrossRef]
- Lee, H.J.; Engelhardt, B.; Lesley, J.; Bickel, U.; Pardridge, W.M. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J. Pharmacol. Exp. Ther. 2000, 292, 1048–1052. [Google Scholar]
- Conaty, P.; Sherman, L.S.; Naaldijk, Y.; Ulrich, H.; Stolzing, A.; Rameshwar, P. Methods of Mesenchymal Stem Cell Homing to the Blood–Brain Barrier. In Somatic Stem Cells; Humana Press: New York, NY, USA, 2018; pp. 81–91. [Google Scholar]
- Atoui, R.; Chiu, R.C.J. Immune Responses after Mesenchymal Stem Cell Implantation. Methods Mol. Biol. 2013, 1036, 107–120. [Google Scholar] [CrossRef]
- Sardesai, V.S.; Shafiee, A.; Fisk, N.M.; Pelekanos, R.A. Avoidance of Maternal Cell Contamination and Overgrowth in Isolating Fetal Chorionic Villi Mesenchymal Stem Cells from Human Term Placenta. STEM CELLS Transl. Med. 2017, 6, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Łęgosz, P.; Sarzyńska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, E.; Brannon, A.L.; Del Vecchio, A.; Garcia, P.E.; Penny, M.K.; Kane, K.T.; Vinta, A.; Buckanovich, R.J.; Di Magliano, M.P. Mesenchymal Stem Cells Promote Pancreatic Tumor Growth by Inducing Alternative Polarization of Macrophages. Neoplasia 2016, 18, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Akers, J.C.; Ramakrishnan, V.; Yang, I.; Hua, W.; Mao, Y.; Carter, B.S.; Chen, C.C. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark. 2016, 17, 125–132. [Google Scholar] [CrossRef] [Green Version]
- De Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marbán, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Marbán, E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu. Rev. Physiol. 2016, 78, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef]
- Deng, M.; Xiao, H.; Peng, H.-L.; Yuan, H.; Xu, Y.; Zhang, G.; Tang, J.; Hu, Z. Preservation of neuronal functions by exosomes derived from different human neural cell types under ischemic conditions. Eur. J. Neurosci. 2017, 47, 150–157. [Google Scholar] [CrossRef]
- Xin, H.; Li, Y.; Liu, Z.; Wang, X.; Shang, X.; Cui, Y.; Zhang, Z.G.; Chopp, M. MiR-133b Promotes Neural Plasticity and Functional Recovery After Treatment of Stroke with Multipotent Mesenchymal Stromal Cells in Rats Via Transfer of Exosome-Enriched Extracellular Particles. Stem Cells 2013, 31, 2737–2746. [Google Scholar] [CrossRef] [Green Version]
- Guitart, K.; Loers, G.; Buck, F.; Bork, U.; Schachner, M.; Kleene, R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 2016, 64, 896–910. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, P.; Liu, S.; Jin, Z.; Li, D.; Zhao, H.; Zhu, X.; Shu, C.; Yan, D.; Dong, Z. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 2019, 28, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2016, 54, 2659–2673. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Liu, R.; Zhu, X.; Smerin, D.; Zhong, Y.; Gu, L.; Fang, W.; Xiong, X. The Involvement and Therapy Target of Immune Cells after Ischemic Stroke. Front. Immunol. 2019, 10, 2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abumiya, T.; Lucero, J.; Heo, J.H.; Tagaya, M.; Koziol, J.A.; Copeland, B.R.; Del Zoppo, G.J. Activated Microvessels Express Vascular Endothelial Growth Factor and Integrin αvβ3During Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 1038–1050. [Google Scholar] [CrossRef] [Green Version]
- Arosio, D.; Casagrande, C. Advancement in integrin facilitated drug delivery. Adv. Drug Deliv. Rev. 2016, 97, 111–143. [Google Scholar] [CrossRef]


| Authors, Year | Species in Experiments In Vivo | Stroke Model, Duration of Ischemia, Min | Therapeutic Intervention by Exosomes, Route of Administration, Timing, Dosage | Source of Exosome | Behavioral Outcome Assessment | Maximum Date of Evaluation for Motor Function |
|---|---|---|---|---|---|---|
| Xin H et al., 2013 [35] | Male Wistar rats (weighing 270–300 g) | tMCAO, 120 | IV injection, 24 h after ischemia, 100 μg | BMSCs | 1. mNSS 2. Foot-fault test | 28 |
| Zhao Y et al., 2020 [36] | Male SD rats (weighing 270 ± 10 g) | tMCAO, 90 | IV injection, 2 h after ischemia, 120 μg | BMSCs | 1. Neurological severity score 2. Shuttle-box test | 7 |
| Doeppner TR et al., 2015 [37] | Male C57BL/6 mice (10 weeks old) | tMCAO, 30 | IV injection, 3 and 5 days after ischemia, 2 x106 MSCs released | BMSCs | 1. Rotarod test 2. Tightrope test 3. Corner turn test | 28 |
| Ling X et al., 2020 [38] | Male SD rats (6–8 weeks old, weighing 250–300 g) | tMCAO, 120 | IV injection, 4 h after ischemia, approximately 1 × 1011 | Urine-derived stem cells | 1. mNSS 2. Foot-fault test | 28 |
| Nalamolu KR et al., 2019 [39] | Male SD rats (weighing 210 ± 10 g) | tMCAO, 120 | IV injection, immediately after reperfusion, 150 μg * | HUCB-MSCs | 1. mNSS 2. Modified adhensive removal test 3. Beam walking 4. Accelerating rotarod performance tests | 7 |
| Nalamolu KR et al., 2019 [40] | Male SD rats (weighing 210 ± 10 g) | tMCAO, 120 | IV injection, immediately after reperfusion, 150 μg ** | HUCB-MSCs | 1. mNSS 2. Modified adhensive removal test 3. Beam walking 4. Accelerating rotarod performance tests | 7 |
| Moon Gj et al., 2019 [41] | Male SD rats (8 weeks old, 270–300 g) | tMCAO, 90 | IV injection, 24 h after ischemia, 30 μg | BMSCs | 1. mNSS 2. Cylinder and ladder rung walking test | 28 |
| Safakheil M et al., 2020 [42] | Male Wistar rats (weighing 280–300 g) | tMCAO, 60 | Stereotaxic administration, 3 h after ischemia; 100 μg, oral gavage, rosuvastatin (20 mg/kg/day); or both | BMSCs | 1. The elevated body swing test 2. Garcia score | 7 |
| Xin H et al., 2017 [43] | Male Wistar rats (weighing 270–300 g) | tMCAO, 120 | IV injection, 24 h after ischemia, 100 μg | BMSCs | 1. mNSS 2. Foot-fault test | 28 |
| Xin H et al., 2017 [44] | Male Wistar rats (weighing 270–300 g) | tMCAO, 120 | IV injection, 24 h after ischemia, 100 μg (comparable to 3 X 1011 particles) | BMSCs | 1. mNSS 2. Foot-fault test | 28 |
| Liu Y et al., 2019 [45] | SD rats (8–12 weeks old, weighting 220–240 g) | tMCAO, 120 | IV injection, 4 or 12 h after ischemia, 0.5 × 105 particles | BMSC | 1. Neurological scores 2. Inclined board test | 21 |
| Geng W et al., 2019 [46] | Male SD rats (weighing 280 ± 10 g) | tMCAO, 120 | IV injection, 24 h after ischemia, exosome pellet in 200 μL, | Rat adipose derived stem cells | 1. mNSS 2. Foot-fault test | 14 |
| Chen KH, et al., 2016 [47] | Male SD rats (weighing 350–375 g) | tMCAO, 50 | IV injection, 3 h after ischemia, 100 μg | ADMSC | 1. Corner test | 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, Y.; Hira, K.; Miyamoto, N.; Kijima, C.; Inaba, T.; Hattori, N. Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery. Int. J. Mol. Sci. 2020, 21, 6894. https://doi.org/10.3390/ijms21186894
Ueno Y, Hira K, Miyamoto N, Kijima C, Inaba T, Hattori N. Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery. International Journal of Molecular Sciences. 2020; 21(18):6894. https://doi.org/10.3390/ijms21186894
Chicago/Turabian StyleUeno, Yuji, Kenichiro Hira, Nobukazu Miyamoto, Chikage Kijima, Toshiki Inaba, and Nobutaka Hattori. 2020. "Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery" International Journal of Molecular Sciences 21, no. 18: 6894. https://doi.org/10.3390/ijms21186894
APA StyleUeno, Y., Hira, K., Miyamoto, N., Kijima, C., Inaba, T., & Hattori, N. (2020). Pleiotropic Effects of Exosomes as a Therapy for Stroke Recovery. International Journal of Molecular Sciences, 21(18), 6894. https://doi.org/10.3390/ijms21186894