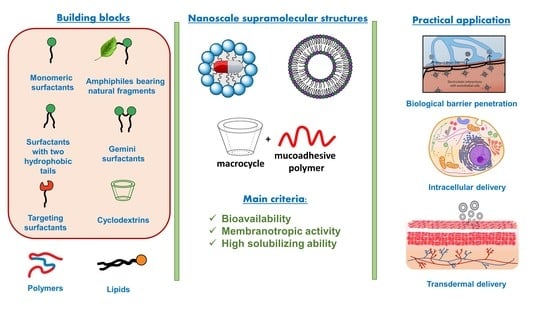
Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers
Abstract
:1. Self-Assembly of Amphiphilic Compounds
1.1. Cationic Surfactants Bearing Cleavable Fragments
1.2. Amphiphilic Compounds with Natural Fragment
1.2.1. Amphiphilic Compounds Bearing Amino Acid Fragments
1.2.2. Sugar-Based Amphiphilic Compounds
1.2.3. Bile Salts and Derivatives of Bile Acids—Natural Amphiphiles and Their Artificially Designed Counterparts
1.2.4. Nucleolipids (Amphiphilic Derivatives of Nucleobases)
1.3. Silicone-Based Surfactants
2. Self-Assembled Amphiphilic Systems as Smart Drug Nanocarriers
2.1. Endosomal Escape
2.2. Liposomal Nanocarriers
2.2.1. Advantages and Limitations of Liposomal Formulations
2.2.2. Cationic Liposomes as Drug Carriers
2.2.3. Cationic Liposomes Obtained by Non-Covalent Modification with Surfactants
2.2.4. pH-Dependent Cationic Lipids
3. Mucoadhesive Properties of Supramolecular Systems Based on Macrocycles and Polymers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CMC | Critical micelle concentration |
| TMA | Trimethylammonium |
| DNA | Deoxyribonucleic acid |
| DOX | Doxorubicin |
| BSA | Bovine serum albumin |
| MCF-7 | Michigan cancer foundation-7 |
| HEK | Human embryonic kidney |
| ATG | Alkyl triazole glycosides |
| TEM | Transmission electron microscopy |
| EGFR | Epidermal growth factor receptor |
| PEI | Polyethylenimine |
| PAMAM | Polyamidoamine |
| TAT | Transactivator of transcription |
| FDA | Food and drug administration |
| RES | Reticuloendothelial system |
| PEG | Polyethylene glycol |
| EPR | Enhanced permeability and retention |
| ABC | Accelerated blood clearance |
| HER2 | Human epidermal growth factor 2 |
| RGD | Arginine-glycine-aspartic acid |
| CL | Cationic liposome |
| siRNA | Small interfering RNA |
| BBB | Blood-brain barrier |
| DPPC | 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine |
| SHP-n-Q | Sterically hindered phenol containing a quaternary ammonium moiety |
| DMPC | 1,2-Dimyristoyl-sn-glycero-3-phosphatidylcholine |
| CTAB | Cetyltrimethylammonium bromide |
| 2-PAM | 2-Pyridine aldoxime methyl chloride |
| PAMPA | Parallel artificial membrane permeability assay |
| hCMEC/D3 | Human cerebral microvascular endothelial cell line, D3 clone |
| DABCO | 1,4-Diazabicyclo [2.2.2]octane |
| DOPC | 1,2-Dioleoyl-sn-glycero-3-phosphocholine |
| DOPG | 1,2-Dioleoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt |
| DC-cholesterol | 3β-[N-(N’,N’-Dimethylaminoethane)-carbamoyl]cholesterol hydrochloride |
| CD | Cyclodextrin |
References
- Rusanov, A.I. Micellization in Surfactant Solutions; Taylor & Francis: London, UK, 1997; 326p. [Google Scholar]
- Zakharova, L.Y.; Pashirova, T.N.; Doktorovova, S.; Fernandes, A.R.; Sanchez-Lopez, E.; Silva, A.M.; Souto, S.B.; Souto, E.B. Cationic surfactants: Self-assembly, structure-activity correlation and their biological applications. Int. J. Mol. Sci. 2019, 20, 5534. [Google Scholar]
- Ghosh, S.; Ray, A.; Pramanik, N. Self-assembly of surfactants: An overview on general aspects of amphiphiles. Biophys. Chem. 2020, 265, 106429. [Google Scholar]
- Ansari, M.J. An overview of techniques for multifold enhancement in solubility of poorly soluble drugs. Curr. Issues Pharm. Med. Sci. 2019, 32, 203–209. [Google Scholar]
- Kwa’sniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens 2020, 9, 459. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar]
- Anestopoulos, I.; Kiousi, D.; Klavaris, E.A.; Galanis, A.; Salek, K.; Euston, S.R.; Pappa, A.; Panayiotidis, M.I. Surface active agents and their health-promoting properties: Molecules of multifunctional significance. Pharmaceutics 2020, 12, 688. [Google Scholar]
- Zhou, C.; Wang, Y. Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 2020, 45, 28–43. [Google Scholar]
- Para, G.; Łuczyn´ski, J.; Palus, J.; Jarek, E.; Wilk, K.A.; Warszyn´ski, P. Hydrolysis driven surface activity of esterquat surfactants. J. Colloid Interface Sci. 2016, 465, 174–182. [Google Scholar]
- Yasa, S.R.; Kaki, S.S.; Poornachandra, Y.; Kumar, C.G.; Penumarthy, V. Synthesis, characterization, antimicrobial and biofilm inhibitory studies of new esterquats. Bioorg. Med. Chem. Lett. 2016, 26, 1978–1982. [Google Scholar]
- Bhat, I.A.; Roy, B.; Kabir-ud-Din. Micelles of cleavable gemini surfactant induce fluorescence switching in novel probe: Industrial insight. J. Ind. Eng. Chem. 2019, 77, 60–64. [Google Scholar]
- Wu, J.; Gao, H.; Shi, D.; Yang, Y.; Zhang, Y.; Zhu, W. Cationic gemini surfactants containing both amide and ester groups: Synthesis, surface properties and antibacterial activity. J. Mol. Liq. 2020, 299, 112248. [Google Scholar]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Voloshina, A.D.; Lenina, O.A.; Zakharova, L.Y.; Sinyashin, O.G. Carbamate-bearing surfactants: Micellization, solubilization, and biological activity. J. Mol. Liq. 2018, 269, 203–210. [Google Scholar]
- Kushnazarova, R.A.; Mirgorodskaya, A.B.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel cationic surfactants bearing cleavable carbamate fragment: Tunable morphological behavior, solubilization of hydrophobic drugs and cellular uptake study. J. Mol. Liq. 2020, 318, 113894. [Google Scholar]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Zakharova, L.Y. Self-assembly of mixed systems based on nonionic and carbamate-bearing cationic surfactants as a tool for fabrication of biocompatible nanocontainers. J. Mol. Liq. 2019, 292, 111407. [Google Scholar]
- Mirgorodskaya, A.B.; Kushnazarova, R.A.; Lukashenko, S.S.; Nikitin, E.N.; Sinyashin, K.O.; Nesterova, L.M.; Zakharova, L.Y. Carbamate-bearing surfactants as effective adjuvants promoted the penetration of the herbicide into the plant. Colloids Surf. A 2020, 586, 124252. [Google Scholar]
- Rusanov, A.I. Micelle Formation in Surfactant Solutions; Chemistry: St. Petersburg, Russia, 1992; 280p. [Google Scholar]
- Bordes, R.; Holmberg, K. Amino acid-based surfactants—Do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91. [Google Scholar]
- Aslam, J.; Lone, I.H.; Radwan, N.R.E.; Siddiqui, M.F.; Parveen, S.; Alnoman, R.B.; Aslam, R. Molecular interaction of amino acid-based gemini surfactant with human serum albumin: Tensiometric, spectroscopic, and molecular docking study. ACS Omega 2019, 4, 22152–22160. [Google Scholar]
- Rostami, A.; Hashemi, A.; Takassi, M.A.; Zadehnazari, A. Experimental assessment of a lysine derivative surfactant for enhanced oil recovery in carbonate rocks: Mechanistic and core displacement analysis. J. Mol. Liq. 2017, 232, 310–318. [Google Scholar]
- Pi-Boleda, B.; Bouzas, M.; Gaztelumendi, N.; Illa, O.; Nogués, C.; Branchadell, V.; Pons, R.; Ortuño, R.M. Chiral pH-Sensitive cyclobutane β-amino acid-based cationic amphiphiles: Possible candidates for use in gene therapy. J. Mol. Liq. 2020, 297, 111856. [Google Scholar]
- Bourkaib, M.C.; Delaunay, S.; Framboisier, X.; Hôtel, L.; Aigle, B.; Humeau, C.; Guiavarc’h, Y.; Chevalot, I. N-acylation of L-amino acids in aqueous media: Evaluation of the catalytic performances of Streptomyces ambofaciens aminoacylases. Enzyme Microb. Technol. 2020, 137, 109536. [Google Scholar]
- Wolf, J.D.; Kurpiers, M.; Baus, R.A.; Götz, R.X.; Griesser, J.; Matuszczak, B.; Bernkop-Schnürch, A. Characterization of an amino acid based biodegradable surfactant facilitating the incorporation of DNA into lipophilic delivery systems. J. Colloid Interface Sci. 2020, 566, 234–241. [Google Scholar]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; Macmillan Publishers Ltd.: New York, NY, USA, 2017; 1312p. [Google Scholar]
- Bernal, C.; Guzman, F.; Illanes, A.; Wilson, L. Selective and eco-friendly synthesis of lipoaminoacid-based surfactants for food, using immobilized lipase and protease biocatalysts. Food Chem. 2018, 239, 189–195. [Google Scholar]
- Katiyar, S.S.; Kushwah, V.; Dora, C.P.; Patil, R.Y.; Jain, S. Design and toxicity evaluation of novel fatty acid-amino acid-based biocompatible surfactants. AAPS PharmSciTech 2019, 20, 186. [Google Scholar]
- Rothbauer, G.A.; Rutter, E.A.; Reuter-Seng, C.; Vera, S.; Billiot, E.J.; Fang, Y.; Billiot, F.H.; Morris, K.F. Nuclear magnetic resonance investigation of the effect of pH on micelle formation by the amino acid-based surfactant undecyl l-phenylalaninate. J. Surfact. Deterg. 2018, 21, 139–153. [Google Scholar]
- Lone, M.S.; Bhat, P.A.; Shah, R.A.; Chat, O.A.; Dar, A.A. A green pH-switchable amino acid based smart wormlike micellar system for efficient and controlled drug delivery. ChemistrySelect 2017, 2, 1144–1148. [Google Scholar]
- Covis, R.; Vives, T.; Gaillard, C.; Benoit, M.; Benvegnu, T. Interactions and hybrid complex formation of anionic algalpolysaccharides with a cationic glycine betaine-derived surfactant. Carbohydr. Polym. 2015, 121, 436–448. [Google Scholar]
- Mondal, S.; Raposo, M.L.; Ghosh, A.; Prieto, G.; Ghosh, S. Physicochemical and conformational studies on interaction of myoglobin with an amino-acid based anionic surfactant, sodium N-dodecanoyl sarcosinate (SDDS). Colloids Surf. A 2019, 577, 167–174. [Google Scholar]
- Lu, H.; Yuan, M.; Fang, B.; Wang, J.; Guo, Y. Wormlike micelles in mixed amino acid-based anionic surfactant and zwitterionic surfactant systems. J. Surfact. Deterg. 2015, 18, 589–596. [Google Scholar]
- Chen, Z.; Zhang, P.; Sun, Y.; Wang, C.; Xu, B. Interfacial dilational rheology of sodium lauryl glycine and mixtures with conventional surfactants. J. Surfact. Deterg. 2019, 22, 1477–1485. [Google Scholar]
- Mustahil, N.A.; Baharuddin, S.H.; Abdullah, A.A.; Reddy, A.V.B.; Mutalib, M.I.A.; Moniruzzaman, M. Synthesis, characterization, ecotoxicity and biodegradability evaluations of novel biocompatible surface active lauroyl sarcosinate ionic liquids. Chemosphere 2019, 229, 349–357. [Google Scholar]
- Sreenu, A.; Nayak, R.R.; Prasad, R.B.N.; Poornachandra, Y.; Kumar, C.G. Surface and antimicrobial properties of N-palmitoyl amino acid-based surfactants. J. Dispers. Sci. Technol. 2015, 36, 765–771. [Google Scholar]
- Perinelli, D.R.; Casettari, L.; Cespi, M.; Fini, F.; Man, D.K.W.; Giorgioni, G.; Canala, S.; Lam, J.K.W.; Bonacucina, G.; Palmieri, G.F. Chemical–physical properties and cytotoxicity of N-decanoyl aminoacid-based surfactants: Effect of polar heads. Colloids Surf. A 2016, 492, 38–46. [Google Scholar]
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Silva, S.G.; do Vale, M.L.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. New serine-derived gemini surfactants as gene delivery systems. Eur. J. Pharm. Biopharm. 2015, 89, 347–356. [Google Scholar]
- Torres, M.A.M.; Gómez, E.M.P.; Fernández, M.A.; Veglia, A.V.; Pacioni, N.L. Role of a cystine-based Gemini surfactant ligand in the synthesis of catalytic active silver nanoparticles. J. Mol. Liq. 2019, 284, 110–116. [Google Scholar]
- Branco, M.A.; Pinheiro, L.; Faustino, C. Amino acid-based cationic gemini surfactant-protein interactions. Colloids Surf. A 2015, 480, 105–112. [Google Scholar]
- Takassi, M.A.; Zargar, G.; Madani, M.; Zadehnazari, A. The preparation of an amino acid-based surfactant and its potential application as an EOR agent. Pet. Sci. Technol. 2017, 35, 385–391. [Google Scholar]
- Pinazo, A.; Petrizelli, V.; Bustelo, M.; Pons, R.; Vinardell, M.P.; Mitjans, M.; Manresa, A.; Perez, L. New cationic vesicles prepared with double chain surfactants from arginine: Role of the hydrophobic group on the antimicrobial activity and cytotoxicity. Colloids Surf. B 2016, 141, 19–27. [Google Scholar]
- Shahzadi, I.; Asim, M.H.; Dizdarevic, A.; Wolf, J.D.; Kurpiers, M.; Matuszczak, B.; Bernkop-Schnürch, A. Arginine-based cationic surfactants: Biodegradable auxiliary agents for the formation of hydrophobic ion pairs with hydrophilic macromolecular drugs. J. Colloid Interface Sci. 2019, 552, 287–294. [Google Scholar]
- Hu, B.; Yuan, Y.; Yan, Y.; Zhou, X.; Li, Y.; Kan, Q.; Li, S. Preparation and evaluation of a novel anticancer drug delivery carrier for 5-Fluorouracil using synthetic bola-amphiphile based on lysine as polar heads. Mater. Sci. Eng. C 2017, 75, 637–645. [Google Scholar]
- Bračič, M.; Fras-Zemljič, L.; Perez, L.; Kogej, K.; Stana-Kleinschek, K.; Kargla, R.; Mohan, T. Protein-repellent and antimicrobial nanoparticle coatings from hyaluronic acid and a lysine-derived biocompatible surfactant. J. Mater. Chem. B 2017, 5, 3888–3897. [Google Scholar]
- Oliveira, I.S.; Lo, M.; Araújo, M.J.; Marques, E.F. Temperature-responsive self-assembled nanostructures from lysine-based surfactants with high chain length asymmetry: From tubules and helical ribbons to micelles and vesicles. Soft Matter 2019, 15, 3700–3711. [Google Scholar]
- Bustelo, M.; Pinazo, A.; Manresa, M.A.; Mitjans, M.; Vinardell, M.P.; Pérez, L. Monocatenary histidine-based surfactants: Role of the alkyl chain length in antimicrobial activity and their selectivity over red blood cells. Colloids Surf. A 2017, 532, 501–509. [Google Scholar]
- Fait, M.E.; Hermet, M.; Vazquez, R.; Mate, S.; Daza Millone, M.A.; Vela, M.E.; Morcelle, S.R.; Bakas, L. Volume expansion of erythrocytes is not the only mechanism responsible for the protection by arginine-based surfactants against hypotonic hemolysis. Colloids Surf. B 2018, 171, 134–141. [Google Scholar]
- Pinazo, A.; Pons, R.; Bustelo, M.; Manresa, M.A.; Morán, C.; Raluy, M.; Pérez, L. Gemini histidine based surfactants: Characterization; surface properties and biological activity. J. Mol. Liq. 2019, 289, 111156. [Google Scholar]
- Barai, M.; Mandal, M.K.; Sultana, H.; Manna, E.; Das, S.; Nag, K.; Ghosh, S.; Patra, A.; Panda, A.K. Theoretical approaches on the synergistic interaction between double-headed anionic amino acid-based surfactants and hexadecyltrimethylammonium bromide. J. Surfactants Deterg. 2020. [Google Scholar] [CrossRef]
- Barai, M.; Mandal, M.K.; Karak, A.; Bordes, R.; Patra, A.; Dalai, S.; Panda, A.K. Interfacial and aggregation behavior of dicarboxylic amino acid based surfactants in combination with a cationic surfactant. Langmuir 2019, 35, 15306–15314. [Google Scholar]
- Zheng, Y.; Zheng, M.; Ma, Z.; Xin, B.; Guo, R.; Xu, X. Sugar fatty acid esters. In Polar Lipids: Biology Chemistry and Technology; Academic Press and AOCS Press: Urbana, Italy, 2015; 568p. [Google Scholar]
- Wang, Y.; Yan, F.; Jia, Q.; Wang, Q. Quantitative structure-property relationship for critical micelles concentration of sugar-based surfactants using norm indexes. J. Mol. Liq. 2018, 253, 205–210. [Google Scholar]
- Ji, S.; Shen, W.; Chen, L.; Zhang, Y.; Wu, X. Synthesis and properties of alkoxyethyl 2-acetamido-2-deoxy-α-D-glucopyranoside. J. Mol. Liq. 2017, 242, 1169–1175. [Google Scholar]
- Li, J.; Bai, Y.; Wang, W.; Tai, X.; Wang, G. Green glucamine-based trisiloxane surfactant: Surface activity, aggregate behavior, and superspreading on hydrophobic surfaces. ACS Sustain. Chem. Eng. 2019, 7, 4390–4398. [Google Scholar]
- Gan, C.; Cai, K.; Qu, X.; Li, H.; Wei, L.; Cheng, R. Glucose-based novel gemini surfactants: Surface activities, aggregation properties and a preliminary study as nanocarrier for resveratrol. J. Mol. Liq. 2019, 283, 781–787. [Google Scholar]
- Sharma, L.; Singh, S.N. Reverse micellar encapsulation of D- and L-enantiomers of some aromatic a-amino acids and nucleobases by glucose-derived non-ionic gemini surfactants in neat n-hexane. J. Surfact. Deterg. 2015, 18, 33–39. [Google Scholar]
- Wu, X.; Chen, L.; Fu, F.; Fan, Y.; Luo, Z. Synthesis and surface properties of alkyl β-D-thioglucopyranoside. J. Mol. Liq. 2019, 276, 282–289. [Google Scholar]
- Fan, L.; Xie, P.; Wang, Y.; Liu, X.; Lia, Y.; Zhou, J. Influences of mannosylerythritol lipid-A on the self-assembling structure formation and functional properties of heat-induced β-lactoglobulin aggregates. Food Hydrocoll. 2019, 96, 310–321. [Google Scholar]
- Shen, W.; Ji, S.; Chen, L.; Zhang, Y.; Wu, X. Synthesis and properties of alkoxyethyl β-D-xylopyranoside. J. Surfact. Deterg. 2018, 21, 255–267. [Google Scholar]
- Ge, X.; Zhang, S.; Chen, X.; Liu, X.; Qian, C. A designed bi-functional sugar-based surfactant: Micellar catalysis for C–X coupling reaction in water. Green Chem. 2019, 21, 2771–2776. [Google Scholar]
- Bettoschi, A.; Brisson, A.; Caltagirone, C.; Falchi, A.M.; Isaia, F.; Lippolis, V.; Loi, G.; Loi, M.; Murgia, S.; Pilia, R.; et al. Fluorescent lactose-derived catanionic aggregates: Synthesis, characterisation and potential use as antibacterial. RSC Adv. 2016, 6, 23340–23344. [Google Scholar]
- Xin, Z.; Du, B.; Yan, S.; Du, S.; Zhao, C.; Sun, M.; Gao, Y. Surface modification of polyurethane via covalent immobilization of sugar-based trisiloxane surfactants. Des. Monomers Polym. 2015, 18, 284–294. [Google Scholar]
- Zhang, L.; Dong, Y.; Zhang, X.; Guo, X. Micellization of lactosylammonium surfactants with different counter ions and their interaction with DNA. J. Chem. Eng. Data 2016, 61, 2969–2978. [Google Scholar]
- Roig, F.; Blanzat, M.; Solans, C.; Esquena, J.; García-Celma, M.J. Hyaluronan based materials with catanionic sugar-derivedsurfactants as drug delivery systems. Colloids Surf. B 2018, 164, 218–223. [Google Scholar]
- Kanan, K.; Fanun, M.; Wadaah, S.; Kayali, I. Formulation of microemulsions based on sugar surfactant as an alternative fuel. J. Dispersion Sci. Technol. 2015, 36, 1009–1014. [Google Scholar]
- Krawczyk, J. Solid wettability modification via adsorption of antimicrobial sucrose fatty acid esters and some other sugar-based surfactants. Molecules 2018, 23, 1597. [Google Scholar]
- Krawczyk, J. Thermodynamic properties of disaccharide based surfactants adsorption at the water-air interface. Colloids Surf. A 2018, 551, 50–57. [Google Scholar]
- Khan, Z.; Al-Thabaiti, S.A.; Malik, M.A. Biocompatible natural sugar-based surfactant assisted oxidation of citric acid by MnO4 in absence and presence of SDS. RSC Adv. 2016, 6, 45993–46001. [Google Scholar]
- Baccile, N.; Griel, P.L.; Prevost, S.; Everaert, B.; Van Bogaert, I.N.A.; Roelants, S.; Soetaert, W. Glucosomes: Glycosylated vesicle-in-vesicle aggregates in water from pH-responsive microbial glycolipid. ChemistryOpen 2017, 6, 526–533. [Google Scholar]
- Carvalho, L.; Morales, J.C.; Perez-Victoria, J.M.; Perez-Victoria, I. Hemolytic activity and solubilizing capacity of raffinose and melezitose fatty acid monoesters prepared by enzymatic synthesis. Eur. J. Pharm. Biopharm. 2015, 92, 139–145. [Google Scholar]
- Ogawa, S.; Kawai, R.; Koga, M.; Asakura, K.; Takahashi, I.; Osanai, S. Oligosaccharide-based surfactant/citric acid buffer system stabilizes lactate dehydrogenase during freeze-drying and storage without the addition of natural sugar. J. Oleo Sci. 2016, 65, 525–532. [Google Scholar]
- Han, N.S.; Heidelberg, T.; Salman, A.A. Spacer effect on triazole-linked sugar-based surfactants. J. Dispersion Sci. Technol. 2016, 38, 105–109. [Google Scholar]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.K.W.; Casettari, L.; Duranti, A. Synthesis, structure–activity relationships and in vitro toxicity profile of lactose-based fatty acid monoesters as possible drug permeability enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar]
- Sandoval-Altamirano, C.; Sánchez, S.A.; Pizarro, N.; Morales, J.; Gunther, G. Curvophilic-curvophobic balance of monoalkyl-mannosides determines the magnitude of disturbance promoted in synthetic bilayers. J. Mol. Liq. 2019, 282, 347–355. [Google Scholar]
- Zdarta, A.; Pacholak, A.; Smułek, W.; Zgoła-Grześkowiak, A.; Ferlin, N.; Bil, A.; Kovensky, J.; Grand, E.; Kaczorek, E. Biological impact of octyl D-glucopyranoside based surfactants. Chemosphere 2019, 217, 567–575. [Google Scholar]
- Feng, J.; Lin, C.; Wang, H.; Liu, S. Gemini dodecyl O-glucoside-based vesicles as nanocarriers for catechin laurate. J. Funct. Foods 2017, 32, 256–265. [Google Scholar]
- Gan, C.; Li, H.; Cai, K. Novel Sugar-Based Gemini Surfactants and Their Surface Properties. J. Surfactants Deterg. 2018, 21, 859–866. [Google Scholar]
- Gan, C.; Wang, H.; Zhao, Z.; Yin, B. Sugar-based ester quaternary ammonium compounds and their surfactant properties. J. Surfactants Deterg. 2014, 17, 465–470. [Google Scholar]
- Chen, L.; Dong, J.; Guo, X. Extraction of bovine serum albumin with reverse micelles from glucosylammonium and lactosylammonium surfactants. Process Biochem. 2017, 60, 108–114. [Google Scholar]
- Parikh, K.; Singh, S.; Kumar, S. Self assembly in an aqueous gemini surfactant containing sugar based (isosorbide) spacer. Arabian J. Chem. 2020, 13, 1848–1857. [Google Scholar]
- Liu, S.; Sang, R.; Hong, S.; Cai, Y.; Wang, H. A novel type of highly effective nonionic gemini alkyl O-glucoside surfactants: A versatile strategy of design. Langmuir 2013, 29, 8511–8516. [Google Scholar]
- Macierzanka, A.; Torcello-Gómez, A.; Jungnickel, C.; Maldonado-Valderrama, J. Bile salts in digestion and transport of lipids. Adv. Colloid Interface Sci. 2019, 274, 102045. [Google Scholar]
- Li, N.; Mosquera-Giraldo, L.I.; Borca, C.H.; Ormes, J.D.; Lowinger, M.; Higgins, J.D.; Slipchenko, L.V.; Taylor, L.S. A comparison of the crystallization inhibition properties of bile salts. Cryst. Growth Des. 2016, 16, 7286–7300. [Google Scholar]
- Faustino, C.; Serafim, C.; Rijo, P.; Pinto Reis, C. Bile acids and bile acid derivatives: Use in drug delivery systems and as therapeutic agents. Expert Opin. Drug Deliv. 2016, 13, 1133–1148. [Google Scholar]
- Alvarez-Figueroa, M.J.; Muggli-Galaz, C.; González, P.M. Effect of the aggregation state of bile salts on their transdermal absorption enhancing properties. J. Drug Deliv. Sci. Technol. 2019, 54, 101333. [Google Scholar]
- Padasala, S.; Patel, V.; Ray, D.; Singh, K.; Aswal, V.K.; Bahadur, P. Bile salt assisted morphological changes of cationic gemini surfactant (12-4-12) micelles. RSC Adv. 2016, 6, 96584–96594. [Google Scholar]
- Roy, A.; Kundu, S.; Dutta, R.; Sarkar, N. Influence of bile salt on vitamin E derived vesicles involving a surface active ionic liquid and conventional cationic micelle. J. Colloid Interface Sci. 2017, 501, 202–214. [Google Scholar]
- Singh, G.; Singh, K.M.; Singh, O.; Kang, T.S. Hydrophobically driven morphologically diverse self-assembled architectures of deoxycholate and imidazolium-based biamphiphilic ionic liquids in aqueous medium. J. Phys. Chem. B 2018, 122, 12227–12239. [Google Scholar]
- Garg, A.; Ali, A.A.; Damarla, K.; Kumar, A.; Sarma, D. Aqueous bile salt accelerated cascade synthesis of 1,2,3-triazoles from arylboronic acids. Tetrahedron Lett. 2018, 59, 4031–4035. [Google Scholar]
- Vinarov, Z.; Katev, V.; Burdzhiev, N.; Tcholakova, S.; Denkov, N. Effect of surfactant-bile interactions on the solubility of hydrophobic drugs in biorelevant dissolution media. Mol. Pharm. 2018, 15, 5741–5753. [Google Scholar]
- Natalini, P.M.; Razuc, M.F.; Sørlic, J.B.; Bucalá, V.; Ramírez-Rigo, M.V. The influence of surfactant on the properties of albendazole-bile salts particles designed for lung delivery. J. Drug Deliv. Sci. Technol. 2019, 53, 101162. [Google Scholar]
- di Gregorio, M.C.; Gubitosi, M.; Travaglini, L.; Pavel, N.V.; Jover, A.; Meijide, F.; Vázquez Tato, J.; Sennato, S.; Schillén, K.; Tranchini, F.; et al. Supramolecular assembly of a thermoresponsive steroidal surfactant with an oppositely charged thermoresponsive block copolymer. Phys. Chem. Chem. Phys. 2017, 19, 1504–1515. [Google Scholar]
- Travaglini, L.; Giordano, C.; D’Annibale, A.; Gubitosi, M.; di Gregorio, M.C.; Schillén, K.; Stefanucci, A.; Mollica, A.; Pavel, N.V.; Galantini, L. Twisted nanoribbons from a RGD-bearing cholic acid derivative. Colloids Surf. B 2017, 159, 183–190. [Google Scholar]
- Chatterjee, S.; Maitra, U. Hierarchical self-assembly of photoluminescent CdS nanoparticles into a bile acid derived organogel: Morphological and photophysical properties. Phys. Chem. Chem. Phys. 2017, 19, 17726–17734. [Google Scholar]
- Arteta, M.Y.; Berti, D.; Montis, C.; Campbell, R.A.; Eriksson, C.; Clifton, L.A.; Skoda, M.W.A.; Soltwedel, O.; Koutsioubas, A.; Baglioni, P.; et al. On the formation of dendrimer/nucleolipids surface films for directed self-assembly. Soft Matter 2015, 11, 1973–1990. [Google Scholar]
- Godeau, G.; Guittard, F.; Darmanin, T. Surfaces bearing fluorinated nucleoperfluorolipids for potential anti-graffiti surface properties. Coatings 2017, 7, 220. [Google Scholar]
- Zhang, D.; Liu, Q.; Visvanathan, R.; Tuchband, M.R.; Sheetah, G.H.; Fairbanks, B.D.; Clark, N.A.; Smalyukh, I.I.; Bowman, C.N. Supramolecular hydrogel prepared from thymine-containing artificial nucleolipid: Study of assembly and lyotropic mesophases. Soft Matter 2018, 14, 7045–7051. [Google Scholar]
- Argudo, P.G.; Muñoz, E.; Giner-Casares, J.J.; Martín-Romero, M.T.; Camacho, L. Folding of cytosine-based nucleolipid monolayer by guanine recognition at the air-water interface. J. Colloid Interface Sci. 2019, 537, 694–703. [Google Scholar]
- Nuthanakanti, A. Cytidine and ribothymidine nucleolipids synthesis, organogelation, and selective anion and metal ion responsiveness. New J. Chem. 2019, 43, 13447–13456. [Google Scholar]
- Chaturvedi, S.; Kaul, A.; Hazari, P.P.; Jha, P.; Pal, S.; Lal, S.; Singh, B.; Barthélémy, P.; Mishra, A.K. Evaluation of BBB permeable nucleolipid (NLDPU): A di-C15-ketalised palmitone appended uridine as neuro-tracer for SPECT. Int. J. Pharm. 2019, 565, 269–282. [Google Scholar]
- Hammerbacher, K.; Görtemaker, K.; Knies, C.; Bender, E.; Bonaterra, G.A.; Rosemeyer, H.; Kinscherf, R. Combinatorial synthesis of new pyrimidine- and purine-ß-D-ribonucleoside nucleolipids: Their distribution between aqueous and organic phases and their in vitro activity against human- and rat glioblastoma cells in vitro. Chem. Biodivers. 2018, 15, e1800173. [Google Scholar]
- Kowouvi, K.; Alies, B.; Gendrot, M.; Gaubert, A.; Vacher, G.; Gaudin, K.; Mosnier, J.; Pradines, B.; Barthelemy, P.; Grislaine, L.; et al. Nucleoside-lipid-based nanocarriers for methylene blue delivery: Potential application as anti-malarial drug. RSC Adv. 2019, 9, 18844–18852. [Google Scholar]
- Gabdrakhmanov, D.R.; Samarkina, D.A.; Semenov, V.E.; Krylova, E.S.; Reznik, V.S.; Zakharova, L.Y. Cationic surfactant with 1,2,4-triazole- and uracil moieties as amphiphilic building blocks for supramolecular nanocontainers. J. Mol. Liq. 2016, 218, 255–259. [Google Scholar]
- Gabdrakhmanov, D.R.; Samarkina, D.A.; Krylova, E.S.; Kapitanov, I.V.; Karpichev, Y.; Latypov, S.K.; Semenov, V.E.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Supramolecular systems based on novel amphiphiles and a polymer: Aggregation and selective solubilization. J. Surfact. Deterg. 2019, 22, 865–874. [Google Scholar]
- Gabdrakhmanov, D.; Samarkina, D.; Semenov, V.; Syakaev, V.; Giniyatullin, R.; Gogoleva, N.; Reznik, V.; Latypov, S.; Konovalov, A.; Pokrovsky, A.; et al. Novel dicationic pyrimidinic surfactant: Self-assembly and DNA complexation. Colloids Surf. A 2015, 480, 113–121. [Google Scholar]
- Gabdrakhamanov, D.R.; Samarkina, D.A.; Semenov, V.E.; Saifina, L.F.; Valeeva, F.G.; Reznik, V.S.; Zakharova, L.Y. Substrate specific nanoreactors based on pyrimidine-containing amphiphiles of various structures for cleavage of phosphonates. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 1673–1675. [Google Scholar]
- Zakharova, L.; Voronin, M.; Semenov, V.; Gabdrakhmanov, D.; Syakaev, V.; Gogolev, Y.; Giniyatullin, R.; Lukashenko, S.; Reznik, V.; Latypov, S.; et al. Supramolecular systems based on novel mono- and dicationic pyrimidinic amphiphiles and oligonucleotides: A self-organization and complexation study. ChemPhysChem 2012, 13, 788–796. [Google Scholar]
- Zakharova, L.Y.; Semenov, V.E.; Voronin, M.A.; Valeeva, F.G.; Kudryavtseva, L.A.; Giniatullin, R.K.; Reznik, V.S.; Konovalov, A.I. Supramolecular catalytic systems based on dimeric pyrimidinic surfactants and polyethyleneimine. Mendeleev Commun. 2008, 18, 158–160. [Google Scholar]
- Gabdrakhmanov, D.R.; Valeeva, F.G.; Zakharova, L.Y.; Giniyatullin, R.K.; Semenov, V.E.; Reznik, V.S.; Konovalov, A.I. Reactivity of phosphorus esters in supramolecular systems based on surfactants containing an uracil residue and polyethylenimine. Russ. J. Org. Chem. 2014, 50, 500–505. [Google Scholar]
- Gabdrakhmanov, D.R.; Kuznetsova, D.A.; Saifina, L.F.; Shulaeva, M.M.; Semenov, V.E.; Zakharova, L.Y. Novel dicationic pyrimidine-based nucleolipid bearing piperidine head groups: Synthesis, aggregation behavior, solubilization capacity and interaction with DNA decamer. Colloids Surf. A 2020, 599, 124853. [Google Scholar]
- Zakharova, L.Y.; Semenov, V.E.; Voronin, M.A.; Valeeva, F.G.; Ibragimova, A.R.; Giniatullin, R.K.; Chernova, A.V.; Kharlamov, S.V.; Kudryavtseva, L.A.; Latypov, S.K.; et al. Nanoreactors based on amphiphilic uracilophanes: Self-organization and reactivity study. J. Phys. Chem. B 2007, 111, 14152–14162. [Google Scholar]
- Gabdrakhmanov, D.R.; Samarkina, D.A.; Semenov, V.E.; Saifina, L.F.; Zakharova, L.Y. Amphiphilic macrocyclic derivative of pyrimidine: Self-assembly, solubilization and interaction with DNA decamer. Macroheterocycles 2017, 10, 567–573. [Google Scholar]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Semenov, V.E.; Valeeva, F.G.; Nikolaev, A.E.; Saifina, L.F.; Zakharova, L.Y. New amphiphilic multiheterocycle: Micelle-forming properties and effect on the reactivity of phosphorus acid esters. Russ. J. Gen. Chem. 2017, 87, 1977–1984. [Google Scholar]
- Ma, J.; Gao, J.; Wang, H.; Lyu, B.; Gao, D. Dissymmetry gemini sulfosuccinate surfactant from vegetable oil: A kind of environmentally friendly fatliquoring agent in the leather industry. ACS Sustain. Chem. Eng. 2017, 5, 10693–10701. [Google Scholar]
- Chen, J.; Mullin, C.A. Characterization of trisiloxane surfactants from agrochemical adjuvants and pollinator-related matrices using liquid chromatography coupled to mass spectrometry. J. Agric. Food Chem. 2015, 63, 5120–5125. [Google Scholar]
- Mao, T.; Wei, Y.; Zheng, C.; Cheng, W.; Zhang, Z.; Zhu, Y.; Wang, R.; Zeng, Z. Antibacterial cotton fabrics coated by biodegradable cationic silicone softeners. J. Surfactants Deterg. 2019, 22, 1429–1443. [Google Scholar]
- Bao, Y.; Guo, J.; Ma, J.; Li, M.; Li, X. Physicochemical and antimicrobial activities of cationic gemini surfactants with polyether siloxane linked group. J. Mol. Liq. 2017, 242, 8–15. [Google Scholar]
- Chen, C.-P.; Lu, F.; Tong, Q.-X. Three tetrasiloxane-tailed cationic gemini surfactants: The effect of different spacer rigidity on surface properties and aggregation behaviors. J. Mol. Liq. 2018, 266, 504–513. [Google Scholar]
- Zhao, X.; Liang, W.; An, D.; Ye, Z. Synthesis and properties of tetrasiloxane Gemini imidazolium surfactants. Colloid Polym. Sci. 2016, 294, 491–500. [Google Scholar]
- Hao, C.; Cui, Y.; Yang, P.; Zhang, H.; Mao, D.; Cui, X.; Li, J. Effect of siloxane spacer length on organosilicon bi-quaternary ammonium amphiphiles. Colloids Surf. B 2015, 128, 528–536. [Google Scholar]
- Tan, J.; Zhao, P.; Ma, D.; Feng, S.; Zhang, C. Effect of hydrophobic chains on the aggregation behavior of cationic silicone surfactants in aqueous solution. Colloid Polym. Sci. 2013, 291, 1487–1494. [Google Scholar]
- Fang, L.; Tan, J.; Zheng, Y.; Yang, G.; Yu, J.; Feng, S. Synthesis, aggregation behavior of novel cationic silicone surfactants in aqueous solution and their application in metal extraction. J. Mol. Liq. 2017, 231, 134–141. [Google Scholar]
- Tan, J.; He, Z.; Miao, Y.; Zhou, D. Effect of steric hindrance on the aggregation behavior of cationic silicone surfactants in aqueous solutions. J. Solution Chem. 2019, 48, 891–904. [Google Scholar]
- Bao, Y.; Guo, J.; Ma, J.; Liu, P.; Kang, Q.; Zhang, J. Cationic silicon-based gemini surfactants: Effect of hydrophobic chains on surface activity, physic-chemical properties and aggregation behaviors. J. Ind. Eng. Chem. 2017, 53, 51–61. [Google Scholar]
- Lin, L.-H.; Wang, C.-C.; Chen, K.-M.; Lin, P.-C. Synthesis and physicochemical properties of silicon-based gemini surfactants. Colloids Surf. A 2013, 436, 881–889. [Google Scholar]
- Huang, Y.; Guo, M.; Tan, J.; Feng, S. Impact of molecular architecture on surface properties and aqueous stabilities of silicone based carboxylate surfactants. Langmuir 2020, 36, 2023–2029. [Google Scholar]
- Naghash, H.J.; Daneshi, M. Synthesis and characterization of a new silicone-based polyurethane surfactant. Polym. Sci., Ser. B 2013, 55, 611–619. [Google Scholar]
- Tan, J.; Liu, Y.; Ye, Z. Synthesis, aggregation behavior of polyether based carbosilane surfactants in aqueous solution. J. Mol. Liq. 2019, 279, 657–661. [Google Scholar]
- Zhou, X.; Lu, H.; Chen, F.; Kong, L.; Zhang, F.; Zhang, W.; Nie, J.; Du, B.; Wang, X. Degradable and thermo-sensitive microgels synthesized via simultaneous quaternization and siloxane condensation. Langmuir 2019, 35, 6145–6153. [Google Scholar]
- Vasilieva, E.A.; Vasileva, L.A.; Valeeva, F.G.; Karimova, T.R.; Zakharov, S.V.; Lukashenko, S.S.; Kuryashov, D.A.; Gaynanova, G.A.; Bashkirtseva, N.Y.; Zakharova, L.Y. Aggregation of pyrrolidinium surfactant in the presence of polymers and hydrotropes. Surf. Innov. 2020, 8, 190–199. [Google Scholar]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Khamatgalimov, A.R.; Kovalenko, V.I.; Zakharova, L.Y. Cationic amphiphiles bearing imidazole fragment: From aggregation properties to potential in biotechnologies. Colloids Surf. A 2017, 529, 990–997. [Google Scholar]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Ahtamyanova, L.R.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Novel hybrid liposomal formulations based on imidazolium-containing amphiphiles for drug encapsulation. Colloids Surf. B 2019, 178, 352–357. [Google Scholar]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Zakharova, L.Y. Self-assembling and biological properties of single-chain dicationic pyridinium-based surfactants. Colloids Surf. B 2019, 175, 351–357. [Google Scholar]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Zakharova, L.Y. N-methyl-D-glucaminocalix[4]resorcinol and its complexes with N-hexadecyl-N’-methyl viologen: Self-assembly and encapsulation activities. Colloids Surf. A 2019, 583, 124033. [Google Scholar]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Kulik, N.V.; Nizameev, I.R.; Kadirov, M.K.; Kashapov, R.R.; Zakharova, L.Y. Supramolecular systems based on cationic imidazole-containing amphiphiles bearing hydroxyethyl fragment: Aggregation properties and functional activity. J. Mol. Liq. 2019, 289, 111058. [Google Scholar]
- Kashapov, R.R.; Razuvayeva, Y.S.; Ziganshina, A.Y.; Mukhitova, R.K.; Sapunova, A.S.; Voloshina, A.D.; Syakaev, V.V.; Latypov, S.K.; Nizameev, I.R.; Kadirov, M.K.; et al. N-methyl-d-glucamine–calix[4]resorcinarene conjugates: Self-assembly and biological properties. Molecules 2019, 24, 1939. [Google Scholar]
- Gabdrakhmanov, D.R.; Valeeva, F.G.; Samarkina, D.A.; Lukashenko, S.S.; Mirgorodskaya, A.B.; Zakharova, L.Y. The first representative of cationic amphiphiles bearing three unsaturated moieties: Self-assembly and interaction with polypeptide. Colloids Surf. A 2018, 558, 463–469. [Google Scholar]
- Samarkina, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Nizameev, I.R.; Kadirov, M.K.; Zakharova, L.Y. Homologous series of amphiphiles bearing imidazolium head group: Complexation with bovine serum albumin. J. Mol. Liq. 2019, 275, 232–240. [Google Scholar]
- Kuznetsova, D.A.; Gabdrakhmanov, D.R.; Lukashenko, S.S.; Voloshina, A.D.; Sapunova, A.S.; Kashapov, R.R.; Zakharova, L.Y. Self-assembled systems based on novel hydroxyethylated imidazolium-containing amphiphiles: Interaction with DNA decamer, protein and lipid. Chem. Phys. Lipids 2019, 223, 104791. [Google Scholar]
- Zakharova, L.Y.; Kaupova, G.I.; Gabdrakhmanov, D.R.; Gaynanova, G.A.; Ermakova, E.A.; Mukhitov, A.R.; Galkina, I.V.; Cheresiz, S.V.; Pokrovsky, A.G.; Skvortsova, P.V.; et al. Alkyl triphenylphosphonium surfactants as nucleic acid carriers: Complexation efficacy toward DNA decamers, interaction with lipid bilayers and cytotoxicity studies. Phys. Chem. Chem. Phys. 2019, 21, 16706–16717. [Google Scholar]
- Gabdrakhmanov, D.R.; Vasilieva, E.A.; Voronin, M.A.; Kuznetsova, D.A.; Valeeva, F.G.; Mirgorodskaya, A.B.; Lukashenko, S.S.; Zakharov, V.M.; Mukhitov, A.R.; Faizullin, D.A.; et al. Soft nanocontainers based on hydroxyethylated geminis: Role of spacer in self-assembling, solubilization, and complexation with oligonucleotide. J. Phys. Chem. C 2020, 124, 2178–2192. [Google Scholar]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale drug delivery systems: From medicine to agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Shmeeda, H.; Zalipsky, S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin. Cancer Res. 2003, 9, 6551–6559. [Google Scholar]
- Hong, S.-S.; Kim, S.H.; Lim, S.-J. Effects of triglycerides on the hydrophobic drug loading capacity of saturated phosphatidylcholine-based liposomes. Int. J. Pharm. 2015, 483, 142–150. [Google Scholar]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar]
- Juang, V.; Chang, C.H.; Wang, C.S.; Wang, H.E.; Lo, Y.L. pH-Responsive PEG-shedding and targeting peptide-modified nanoparticles for dual-delivery of irinotecan and microRNA to enhance tumor-specific therapy. Small 2019, 15, 1–18. [Google Scholar]
- Rayamajhi, S.; Marchitto, J.; Nguyen, T.D.T.; Marasini, R.; Celia, C.; Aryal, S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf. B 2020, 188, 110804. [Google Scholar]
- Fuentes, G.V.; Doucet, E.N.; Abraham, A.; Rodgers, N.K.; Alonso, F.; Euceda, N.; Quinones, M.H.; Riascos, P.A.; Pierre, K.; Sarker, N.H.; et al. Nanocomposite liposomes for pH-controlled porphyrin release into human prostate cancer cells. RSC Adv. 2020, 10, 17094–17100. [Google Scholar]
- Liang, X.; Gao, J.; Jiang, L.; Luo, J.; Jing, L.; Li, X.; Jin, Y.; Dai, Z. Nanohybrid liposomal cerasomes with good physiological stability and rapid temperature responsiveness for high intensity focused ultrasound triggered local chemotherapy of cancer. ACS Nano 2015, 9, 1280–1293. [Google Scholar]
- Hayashi, K.; Watanabe, M.; Iwasaki, T.; Shudou, M.; Uda, R.M. Endosomal escape by photo-activated fusion of liposomes containing a malachite green derivative: A novel class of photoresponsive liposomes for drug delivery vehicles. Photochem. Photobiol. Sci. 2019, 18, 1471–1478. [Google Scholar]
- Lale, S.V.; Kumar, A.; Naz, F.; Bharti, A.C.; Koul, V. Multifunctional ATRP based pH responsive polymeric nanoparticles for improved doxorubicin chemotherapy in breast cancer by proton sponge effect/endo-lysosomal escape. Polym. Chem. 2015, 6, 2115–2132. [Google Scholar]
- Zhao, H.; Li, Q.; Hong, Z. Paclitaxel-loaded mixed micelles enhance ovarian cancer therapy through extracellular pH-triggered PEG detachment and endosomal escape. Mol. Pharm. 2016, 13, 2411–2422. [Google Scholar]
- Pawar, S.; Shevalkar, G.; Vavia, P. Glucosamine-anchored doxorubicin-loaded targeted nano-niosomes: Pharmacokinetic, toxicity and pharmacodynamic evaluation. J. Drug Target. 2016, 24, 730–743. [Google Scholar]
- Natarajan, J.; Baskaran, M.; Humtsoe, L.C.; Vadivelan, R.; Justin, A. Enhanced brain targeting efficacy of Olanzapine through solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 364–371. [Google Scholar]
- Hafez, I.M.; Maurer, N.; Cullis, P.R. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001, 8, 1188–1196. [Google Scholar]
- Guo, S.; Huang, L. Nanoparticles escaping RES and endosome: Challenges for siRNA delivery for cancer therapy. J. Nanomater. 2011, 2011, 742895. [Google Scholar]
- Ahmad, A.; Khan, J.M.; Haque, S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie 2019, 160, 61–75. [Google Scholar]
- Pichon, C.; Billiet, L.; Midoux, P. Chemical vectors for gene delivery: Uptake and intracellular trafficking. Curr. Opin. Biotechnol. 2010, 21, 640–645. [Google Scholar]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garrett, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schätzlein, A.G.; et al. Oral particle uptake and organ targeting drives the activity of amphotericin B nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar]
- Muankaew, C.; Jansook, P.; Sigurđsson, H.H.; Loftsson, T. Cyclodextrin-based telmisartan ophthalmic suspension: Formulation development for water-insoluble drugs. Int. J. Pharm. 2016, 507, 21–31. [Google Scholar]
- Jansook, P.; Kulsirachote, P.; Loftsson, T. Cyclodextrin solubilization of celecoxib: Solid and solution state characterization. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 75–88. [Google Scholar]
- Jansook, P.; Kulsirachote, P.; Asasutjarit, R.; Loftsson, T. Development of celecoxib eye drop solution and microsuspension: A comparative investigation of binary and ternary cyclodextrin complexes. Carbohydr. Polym. 2019, 225, 115209. [Google Scholar]
- Kontogiannidou, E.; Ferrari, M.; Deligianni, A.-D.; Bouropoulos, N.; Andreadis, D.A.; Sorrenti, M.; Catenacci, L.; Nazari, K.; Arshad, M.S.; Chang, M.-W.; et al. In Vitro and Ex Vivo Evaluation of tablets containing piroxicam-cyclodextrin complexes for buccal delivery. Pharmaceutics 2019, 11, 398. [Google Scholar]
- Ahmed, A.; Yadav, H.K.S.; Manne, N.; Sureddy, V.L.; Namburi, N.B.V.; Shivakumar, H.G. Formulation and evaluation of enteric coated nanoparticulate system for poorly absorbable drug. J. Drug. Deliv. Sci. Technol. 2014, 24, 50–56. [Google Scholar]
- Sajomsang, W.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Chaleawlert-umpon, S.; Pimpha, N.; Sramala, I.; Soottitantawat, A.; Puttipipatkhachorn, S.; Ruktanonchai, U.R. A comparison of spacer on water-soluble cyclodextrin grafted chitosan inclusion complex as carrier of eugenol to mucosae. Carbohydr Polym. 2013, 92, 321–327. [Google Scholar]
- Sayed, S.; Elsayed, I.; Ismail, M.M. Optimization of β-cyclodextrin consolidated micellar dispersion for promoting the transcorneal permeation of a practically insoluble drug. Int. J. Pharm. 2018, 549, 249–260. [Google Scholar]
- Maged, A.; Mahmoud, A.A.; Ghorab, M.M. Nano spray drying technique as a novel approach to formulate stable econazole nitrate nanosuspension formulations for ocular use. Mol Pharm. 2016, 13, 2951–2965. [Google Scholar]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Panomsuk, S.; Kaomongkolgit, R.; Opanasopit, P. Fabrication of mucoadhesive chitosan coated polyvinylpyrrolidone/cyclodextrin/clotrimazole sandwich patches for oral candidiasis. Carbohydr. Polym. 2015, 132, 173–179. [Google Scholar]
- Ünal, H.; d’Angelo, I.; Pagano, E.; Borrelli, F.; Izzo, A.; Ungaro, F.; Quaglia, F.; Bilensoy, E. Core–shell hybrid nanocapsules for oral delivery of camptothecin: Formulation development, in vitro and in vivo evaluation. J. Nanopart. Res. 2015, 17, 42. [Google Scholar]
- Rodríguez, I.; Vázquez, J.A.; Pastrana, L.; Khutoryanskiy, V.V. Enhancement and inhibition effects on the corneal permeability of timolol maleate: Polymers, cyclodextrins and chelating agents. Int. J. Pharm. 2017, 529, 168–177. [Google Scholar]
- Zhang, Q.; Neoh, K.G.; Xu, L.; Lu, S.; Kang, E.T.; Mahendran, R.; Chiong, E. Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy. Langmuir 2014, 30, 6151–6161. [Google Scholar]
- Mazzaferro, S.; Bouchemal, K.; Skanji, R.; Gueutin, C.; Chacun, H.; Ponchel, G. Intestinal permeation enhancement of docetaxel encapsulated into methyl-β-cyclodextrin/poly(isobutylcyanoacrylate) nanoparticles coated with thiolated chitosan. J. Control. Release 2012, 162, 568–574. [Google Scholar]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar]
- Stewart, M.P.; Lorenz, A.; Dahlman, J.; Sahay, G. Challenges in carrier-mediated intracellular delivery: Moving beyond endosomal barriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 465–478. [Google Scholar]
- Kumari, S.; MG, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar]
- Conner, S.; Schmid, S. Regulated portals of entry into the cell. Nature. 2003, 422, 37–44. [Google Scholar]
- Tros de Ilarduya, C.; Düzgüneş, N. Delivery of therapeutic nucleic acids via transferrin and transferrin receptors: Lipoplexes and other carriers. Expert Opin Drug Deliv. 2013, 10, 1583–1591. [Google Scholar]
- Watanabe, K.; Kaneko, M.; Maitani, Y. Functional coating of liposomes using a folate–polymer conjugate to target folate receptors. Int. J. Nanomed. 2012, 7, 3679–3688. [Google Scholar]
- Wang, Y.; Zhou, J.; Qiu, L.; Wang, X.; Chen, L.; Liu, T.; Di, W. Cisplatin-alginate conjugate liposomes for targeted delivery to EGFR-positive ovarian cancer cells. Biomaterials 2014, 35, 4297–4309. [Google Scholar]
- Hoyer, J.; Neundorf, I. Peptide vectors for the nonviral delivery of nucleic acids. Acc. Chem. Res. 2012, 45, 1048–1056. [Google Scholar]
- Lia, W.; Nicol, F.; Francis, C.S., Jr. GALA: A designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 967–985. [Google Scholar]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar]
- Elimam, H.; El-Say, K.M.; Cybulsky, A.V.; Khalil, H. Regulation of Autophagy Progress via Lysosomal Depletion by Fluvastatin Nanoparticle Treatment in Breast Cancer Cells. ACS Omega 2020, 5, 15476–15486. [Google Scholar]
- Wei, Y.; Tang, T.; Pang, H. Cellular internalization of bystander nanomaterial induced by TAT-nanoparticles and regulated by extracellular cysteine. Nat. Commun. 2019, 10, 3646–36181. [Google Scholar]
- Qiu, J.; Xia, Y. Killing cancer cells by rupturing their lysosomes. Nat. Nanotechnol. 2020, 15, 252–253. [Google Scholar]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar]
- Hatakeyama, H.; Akita, H.; Harashima, H. The Polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar]
- Yue, X.; Dai, Z. Recent advances in liposomal nanohybrid cerasomes as promising drug nanocarriers. Adv. Colloid Interface Sci. 2014, 207, 32–42. [Google Scholar]
- Teixeira, M.C.; Carbone, C.; Souto, E.B. Beyond liposomes: Recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog. Lipid Res. 2017, 68, 1–11. [Google Scholar]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar]
- Caillaud, M.; El Madani, M.; Massaad-Massade, L. Small interfering RNA from the lab discovery to patients’ recovery. J. Control. Release 2020, 321, 616–628. [Google Scholar]
- Yang, K.; Clifton, G.L.; Hayes, R.L. Gene therapy for central nervous system injury: The use of cationic liposomes: An invited review. J. Neurotrauma 1997, 14, 281–297. [Google Scholar]
- Yuan, X.; Qin, B.; Yin, H.; Shi, Y.; Jiang, M.; Luo, L.; Luo, Z.; Zhang, J.; Li, X.; Zhu, C.; et al. Virus-like nonvirus cationic liposome for efficient gene delivery via endoplasmic reticulum pathway. ACS Cent. Sci. 2020, 6, 174–188. [Google Scholar]
- Yu, S.; Bi, X.; Yang, L.; Wu, S.; Yu, Y.; Jiang, B.; Zhang, A.; Lan, K.; Duan, S. Co-delivery of paclitaxel and PLK1-targeted siRNA using aptamer-functionalized cationic liposome for synergistic anti-breast cancer effects in vivo. J. Biomed. Nanotechnol. 2019, 15, 1135–1148. [Google Scholar]
- Seraj, S.; Lee, J.; Ahn, H.J. Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome: Long-term silencing and anticancer effects in vivo. Biochem. Pharmacol. 2019, 166, 192–202. [Google Scholar]
- Zhang, H.; Yu, N.; Chen, Y.; Yan, K.; Wang, X. Cationic liposome codelivering PI3K pathway regulator improves the response of BRCA1-deficient breast cancer cells to PARP1 inhibition. J. Cell. Biochem. 2019, 120, 13037–13045. [Google Scholar]
- Zhang, C.; Zhang, S.; Zhi, D.; Zhao, Y.; Cui, S.; Cui, J. Co-delivery of paclitaxel and survivin siRNA with cationic liposome for lung cancer therapy. Colloids Surf. A 2020, 585, 124054. [Google Scholar]
- Christensen, D.; Korsholm, K.S.; Rosenkrands, I.; Lindenstrøm, T.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2007, 6, 785–796. [Google Scholar]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. [Google Scholar]
- Knudsen, K.B.; Northeved, H.; Pramod Kumar, E.K.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 467–477. [Google Scholar]
- Yan, X.; Scherphof, G.L.; Kamps, J.A.A.M. Liposome opsonization. J. Liposome Res. 2005, 15, 109–139. [Google Scholar]
- Akita, H.; Kudo, A.; Minoura, A.; Yamaguti, M.; Khalil, I.A.; Moriguchi, R.; Masuda, T.; Danev, R.; Nagayama, K.; Kogure, K.; et al. Multi-layered nanoparticles for penetrating the endosome and nuclear membrane via a step-wise membrane fusion process. Biomaterials 2009, 30, 2940–2949. [Google Scholar]
- Trementozzi, A.N.; Imam, Z.I.; Mendicino, M.; Hayden, C.C.; Stachowiak, J.C. Liposome-mediated chemotherapeutic delivery is synergistically enhanced by ternary lipid compositions and cationic lipids. Langmuir 2019, 35, 12532–12542. [Google Scholar]
- Zakharova, L.Y.; Kashapov, R.R.; Pashirova, T.N.; Mirgorodskaya, A.B.; Sinyashin, O.G. Self-assembly strategy for the design of soft nanocontainers with controlled properties. Mendeleev Commun. 2016, 26, 457–468. [Google Scholar]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics 2019, 11, 1–20. [Google Scholar]
- Ingvarsson, P.T.; Rasmussen, I.S.; Viaene, M.; Irlik, P.J.; Nielsen, H.M.; Foged, C. The surface charge of liposomal adjuvants is decisive for their interactions with the Calu-3 and A549 airway epithelial cell culture models. Eur. J. Pharm. Biopharm. 2014, 87, 480–488. [Google Scholar]
- Tada, R.; Hidaka, A.; Iwase, N.; Takahashi, S.; Yamakita, Y.; Iwata, T.; Muto, S.; Sato, E.; Takayama, N.; Honjo, E.; et al. Intranasal immunization with dotap cationic liposomes combined with DC-cholesterol induces potent antigen-specific mucosal and systemic immune responses in mice. PLoS ONE 2015, 10, 1–21. [Google Scholar]
- Ghaffar, K.A.; Marasini, N.; Giddam, A.K.; Batzloff, M.R.; Good, M.F.; Skwarczynski, M.; Toth, I. Liposome-based intranasal delivery of lipopeptide vaccine candidates against group A streptococcus. Acta Biomater. 2016, 41, 161–168. [Google Scholar]
- Pavlov, R.V.; Gaynanova, G.A.; Kuznetsova, D.A.; Vasileva, L.A.; Zueva, I.V.; Sapunova, A.S.; Buzyurova, D.N.; Babaev, V.M.; Voloshina, A.D.; Lukashenko, S.S.; et al. Biomedical potentialities of cationic geminis as modulating agents of liposome in drug delivery across biological barriers and cellular uptake. Int. J. Pharm. 2020, 587, 119640. [Google Scholar]
- Chen, Y.; Qiao, F.; Fan, Y.; Han, Y.; Wang, Y. Interactions of phospholipid vesicles with cationic and anionic oligomeric surfactants. J. Phys. Chem. B 2017, 121, 7122–7132. [Google Scholar]
- Chen, Y.; Qiao, F.; Fan, Y.; Han, Y.; Wang, Y. Interactions of cationic/anionic mixed surfactant aggregates with phospholipid vesicles and their skin penetration ability. Langmuir 2017, 33, 2760–2769. [Google Scholar]
- Zhang, N.; Qi, R.; Li, H.; Guan, B.; Liu, Y.; Han, Y.; Wang, Y. Interaction of phospholipid vesicles with gemini surfactants of different lysine spacer lengths. Soft Matter 2019, 15, 9458–9467. [Google Scholar]
- Pashirova, T.N.; Zhukova, N.A.; Lukashenko, S.S.; Valeeva, F.G.; Burilova, E.A.; Sapunova, A.S.; Voloshina, A.D.; Mirgorodskaya, A.B.; Zakharova, L.Y.; Sinyashin, O.G.; et al. Multi-targeted approach by 2-benzimidazolylquinoxalines-loaded cationic arginine liposomes against cervical cancer cells in vitro. Colloids Surf. B 2019, 178, 317–328. [Google Scholar]
- Mirgorodskaya, A.B.; Kuznetsova, D.A.; Kushnazarova, R.A.; Gabdrakhmanov, D.R.; Zhukova, N.A.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Sinyashin, O.G.; Mamedov, V.A.; et al. Soft nanocarriers for new poorly soluble conjugate of pteridine and benzimidazole: Synthesis and cytotoxic activity against tumor cells. J. Mol. Liq. 2020, 317, 114007–114015. [Google Scholar]
- Kuznetsova, D.; Gaynanova, G.; Vasileva, L.; Sibgatullina, G.; Samigullin, D.; Sapunova, A.; Voloshina, A.; Galkina, I.; Petrov, K.; Zakharova, L. Mitochondria-targeted cationic liposomes modified with alkyltriphenylphosphonium bromides loaded with hydrophilic drugs: Preparation, cytotoxicity and colocalization assay. J. Mater. Chem. B 2019, 7, 7351–7362. [Google Scholar]
- Pashirova, T.N.; Burilova, E.A.; Tagasheva, R.G.; Zueva, I.V.; Gibadullina, E.M.; Nizameev, I.R.; Sudakov, I.A.; Vyshtakalyuk, A.B.; Voloshina, A.D.; Kadirov, M.K.; et al. Delivery nanosystems based on sterically hindered phenol derivatives containing a quaternary ammonium moiety: Synthesis, cholinesterase inhibition and antioxidant activity. Chem. Biol. Interact. 2019, 310, 108753. [Google Scholar]
- Moyá, M.L.; López-López, M.; Lebrón, J.A.; Ostos, F.J.; Pérez, D.; Camacho, V.; Beck, I.; Merino-Bohórquez, V.; Camean, M.; Madinabeitia, N.; et al. Preparation and characterization of new liposomes. Bactericidal activity of cefepime encapsulated into cationic liposomes. Pharmaceutics 2019, 11, 1–12. [Google Scholar]
- Bombelli, C.; Bordi, F.; Ferro, S.; Giansanti, L.; Jori, G.; Mancini, G.; Mazzuca, C.; Monti, D.; Ricchelli, F.; Sennato, S.; et al. New cationic liposomes as vehicles of m-tetrahydroxyphenylchlorin in photodynamic therapy of infectious diseases. Mol. Pharm. 2008, 5, 672–679. [Google Scholar]
- Sivaramakrishna, D.; Prasad, M.D.; Swamy, M.J. A homologous series of apoptosis-inducing N-acylserinols: Thermotropic phase behavior, interaction with cholesterol and characterization of cationic N-myristoylserinol-cholesterol-CTAB niosomes. Biochim. Biophys. Acta Biomembr. 2019, 1861, 504–513. [Google Scholar]
- Jo, M.; Park, K.M.; Park, J.Y.; Yu, H.; Choi, S.J.; Chang, P.S. Microfluidic assembly of mono-dispersed liposome and its surface modification for enhancing the colloidal stability. Colloids Surf. A 2020, 586, 124202. [Google Scholar]
- Lin, H.W.; Xie, Q.C.; Huang, X.; Ban, J.F.; Wang, B.; Wei, X.; Chen, Y.Z.; Lu, Z.F. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int. J. Nanomed. 2018, 13, 831–842. [Google Scholar]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanić, Ž.; Škalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar]
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf. B 2018, 171, 358–367. [Google Scholar]
- Piazzini, V.; Landucci, E.; Graverini, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Stealth and cationic nanoliposomes as drug delivery systems to increase andrographolide BBB permeability. Pharmaceutics 2018, 10, 1–19. [Google Scholar]
- Rukavina, Z.; Šegvić Klarić, M.; Filipović-Grčić, J.; Lovrić, J.; Vanić, Ž. Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphyloccocus aureus (MRSA) infections. Int. J. Pharm. 2018, 553, 109–119. [Google Scholar]
- Pashirova, T.N.; Sapunova, A.S.; Lukashenko, S.S.; Burilova, E.A.; Lubina, A.P.; Shaihutdinova, Z.M.; Gerasimova, T.P.; Kovalenko, V.I.; Voloshina, A.D.; Souto, E.B.; et al. Synthesis, structure-activity relationship and biological evaluation of tetracationic gemini Dabco-surfactants for transdermal liposomal formulations. Int. J. Pharm. 2020, 575, 118953. [Google Scholar]
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252. [Google Scholar]
- Stefanutti, E.; Papacci, F.; Sennato, S.; Bombelli, C.; Viola, I.; Bonincontro, A.; Bordi, F.; Mancini, G.; Gigli, G.; Risuleo, G. Cationic liposomes formulated with DMPC and a gemini surfactant traverse the cell membrane without causing a significant bio-damage. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2646–2655. [Google Scholar]
- Bombelli, C.; Stringaro, A.; Borocci, S.; Bozzuto, G.; Colone, M.; Giansanti, L.; Sgambato, R.; Toccaceli, L.; Mancini, G.; Molinari, A. Efficiency of liposomes in the delivery of a photosensitizer controlled by the stereochemistry of a gemini surfactant component. Mol. Pharm. 2010, 7, 130–137. [Google Scholar]
- Giansanti, L.; Condello, M.; Altieri, B.; Galantini, L.; Meschini, S.; Mancini, G. Influence of lipid composition on the ability of liposome loaded voacamine to improve the reversion of doxorubicin resistant osteosarcoma cells. Chem. Phys. Lipids 2019, 223, 104781. [Google Scholar]
- Giansanti, L.; Bozzuto, G.; Fracassi, A.; Bombelli, C.; Stringaro, A.; Molinari, A.; Piozzi, A.; Sennato, S.; Mancini, G. Effect of preparation protocol on physicochemical features and biointeractions of pegylated liposomes. Colloids Surf. A 2017, 532, 444–450. [Google Scholar]
- Gao, W.; Chan, J.M.; Farokhzad, O.C. pH-Responsive nanoparticles for drug delivery. Mol. Pharm. 2010, 7, 1913–1920. [Google Scholar]
- Yatvin, M.B.; Kreutz, W.; Horwitz, B.A.; Shinitzky, M. pH-sensitive liposomes: Possible clinical implications. Science 1980, 210, 1253–1255. [Google Scholar]
- Connor, J.; Yatvin, M.B.; Huang, L. pH-sensitive liposomes: Acid-induced liposome fusion. Proc. Natl. Acad. Sci. USA 1984, 81, 1715–1718. [Google Scholar]
- Hu, F.; Yue, H.; Lu, T.; Ma, G. Cytosolic delivery of HBsAg and enhanced cellular immunity by pH-responsive liposome. J. Control. Release 2020, 324, 460–470. [Google Scholar]
- Hafez, I.M.; Ansell, S.; Cullis, P.R. Tunable pH-sensitive liposomes composed of mixtures of cationic and anionic lipids. Biophys. J. 2000, 79, 1438–1446. [Google Scholar]
- Griffiths, J.R. Are cancer cells acidic? Br. J. Cancer 1991, 64, 425–427. [Google Scholar]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar]
- Méndez-Ardoy, A.; Guilloteau, N.; Di Giorgio, C.; Vierling, P.; Santoyo-González, F.; Ortiz Mellet, C.; García Fernández, J.M. β-Cyclodextrin-based polycationic amphiphilic “click” clusters: Effect of structural modifications in their DNA complexing and delivery properties. J. Org. Chem. 2011, 76, 5882–5894. [Google Scholar]
- Tan, H.; Qin, F.; Chen, D.; Han, S.; Lu, W.; Yao, X. Study of glycol chitosan-carboxymethyl β-cyclodextrins as anticancer drugs carrier. Carbohydr. Polym. 2013, 93, 679–685. [Google Scholar]
- Tan, H.; Xue, Y.; Luana, Q.; Yao, X. Evaluation of glycol chitosan-graft-carboxymethyl β-cyclodextrin as potential pH-sensitive anticancer drug carrier by surface plasmon resonance. Anal. Methods. 2012, 4, 2784–2790. [Google Scholar]
- Gonil, P.; Sajomsang, W.; Rungsardthong, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Novel quaternized chitosan containing β-cyclodextrin moiety: Synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2011, 83, 905–913. [Google Scholar]
- Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-umpon, S.; Puttipipatkhachorn, S. Self-aggregates formation and mucoadhesive property of water-soluble β-cyclodextrin grafted with chitosan. Int. J. Biol. Macromol. 2011, 48, 589–595. [Google Scholar]
- Chaleawlert-umpon, S.; Nuchuchua, O.; Saesoo, S.; Gonil, P.; Ruktanonchai, U.R.; Sajomsang, W.; Pimpha, N. Effect of citrate spacer on mucoadhesive properties of a novel water-soluble cationic β-cyclodextrin-conjugated chitosan. Carbohydr. Polym. 2011, 84, 186–194. [Google Scholar]
- Jaiswal, M.; Kumar, M.; Pathak, K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surf. B. 2015, 130, 23–30. [Google Scholar]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar]
- Boya, V.N.; Lovett, R.; Setua, S.; Gandhi, V.; Nagesh, P.K.B.; Khan, S.; Jaggi, M.; Yallapu, M.M.; Chauhan, S.C. Probing mucin interaction behavior of magnetic nanoparticles. J. Colloid Interface Sci. 2017, 488, 258–268. [Google Scholar]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast-acting clotrimazole composited PVP/HPβCD nanofibers for oral candidiasis application. Pharm Res. 2014, 31, 1893–1906. [Google Scholar]
- Kawano, Y.; Imamura, A.; Nakamura, T.; Akaishi, M.; Satoh, M.; Hanawa, T. Development and characterization of oral spray for stomatitis containing irsogladine maleate. Chem. Pharm. Bull. 2016, 64, 1659–1665. [Google Scholar]
- Jha, R.K.; Tiwari, S.; Mishra, B. Bioadhesive microspheres for bioavailability enhancement of raloxifene hydrochloride: Formulation and pharmacokinetic evaluation. AAPS PharmSciTech 2011, 12, 650–657. [Google Scholar]
- Cho, H.; Oh, D.; Kim, D. Polysaccharides-based spray-dried microspheres for maintained stability and controlled release of protein. Int. J. Pharm. Investig. 2012, 42, 83–88. [Google Scholar]
- Zhao, L.; Tang, B.; Tang, P.; Sun, Q.; Suo, Z.; Zhang, M.; Gan, N.; Yang, H.; Li, H. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles for ibrutinib delivery: A potential nanoformulation of novel kinase inhibitor. J. Pharm. Sci. 2020, 109, 1136–1144. [Google Scholar]
- Sampathkumar, K.; Loo, S.C.J. Targeted gastrointestinal delivery of nutraceuticals with polysaccharide-based coatings. Macromol Biosci. 2018, 18, 1700363. [Google Scholar]
- Bal, T.; Murthy, P.N.; Sengupta, S. Preparation and evaluation of mucoadhesive simvastatin microcapsules using orifice gelation technique. Asian J. Pharm. 2012, 6, 74–84. [Google Scholar]
- Nogueiras-Nieto, L.; Sobarzo-Sánchez, E.; Gómez-Amoza, J.L.; Otero-Espinar, F.J. Competitive displacement of drugs from cyclodextrin inclusion complex by polypseudorotaxane formation with poloxamer: Implications in drug solubilization and delivery. Eur. J. Pharm. Biopharm. 2012, 80, 585–595. [Google Scholar]
- Jansook, P.; Pichayakorn, W.; Muankaew, C.; Loftsson, T. Cyclodextrin-poloxamer aggregates as nanocarriers in eye drop formulations: Dexamethasone and amphotericin B. Drug Dev. Ind. Pharm. 2016, 42, 1446–1454. [Google Scholar]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int. J. Pharm. 2011, 413, 229–236. [Google Scholar]
- Galus, A.; Mallet, J.-M.; Lembo, D.; Cagno, V.; Djabourov, M.; Lortat-Jacob, H.; Bouchemal, K. Hexagonal-shaped chondroitin sulfate self-assemblies have exalted anti-HSV-2 activity. Carbohydr. Polym. 2016, 136, 113–120. [Google Scholar]
- Grisin, T.; Bories, C.; Bombardi, M.; Loiseau, P.M.; Rouffiac, V.; Solgadi, A.; Mallet, J.-M.; Ponchel, G.; Bouchemal, K. Supramolecular chitosan micro-platelets synergistically enhance anti-candida albicans activity of amphotericin B using an immunocompetent murine model. Pharm. Res. 2017, 34, 1067–1082. [Google Scholar]
- Carn, F.; Nowak, S.; Chaab, I.; Diaz-Salmeron, R.; Djabourov, M.; Bouchemal, K. Autoassemblies of α-cyclodextrin and grafted polysaccharides: Crystal structure and specific properties of the platelets. J. Phys. Chem. B. 2018, 122, 6055–6063. [Google Scholar]
- Mahlert, L.; Anderski, J.; Mulac, D.; Langer, K. The impact of gastrointestinal mucus on nanoparticle penetration–in vitro evaluation of mucus-penetrating nanoparticles for photodynamic therapy. Eur. J. Pharm. Sci. 2019, 133, 28–39. [Google Scholar]















| Chemical Structure | CMC, mM | MIC, μM |
|---|---|---|
 | 0.7 [47] | 5.0 [47] |
 | 0.3 [40] | 17 [40] |
 | 1.5 [45] | 35 [45] |
 | 1.0 [33] | 450 [33] |
 | 1.0 [33] | 450 [33] |
| Chemical Structure | CMC, μM |
|---|---|
 | 29.0 [80] |
 | 33.0 [76] |
 | 8.0 [69] |
 | 9.0 [69] |
| Chemical Structure | CMC, mM | 103·S, moldye/molamphiphille |
|---|---|---|
 | 15.0 [106] | - |
 | 3.0 [107] | - |
 | 0.05 [108] | - |
 | >100 [106] | |
 | 3.0 [102] | 1.6 [102] |
 | 1.9 [103] | 9.4 [103] |
 | 2.0 [109] | 21 [109] |
 | 3.4 [104] | 1.4 [104] |
 | 0.9 [110] | - |
 | 1.0 [111] | 7.8 [111] |
 | 0.4 [112] | 12.9 [112] |
| Formulation Description | Problem to Be Solved | Solution | Ref |
|---|---|---|---|
| DOX liposomes | fast clearance by the reticuloendothelial system | Modification of liposomes with PEG-conjugated lipids, which hinder their recognition by macrophages. This provides prolonged circulation time of pegylated liposomes. | [142] |
| DOX liposomes | passively targeted versus ligand-targeted liposomes testing | Liposomes modified by folate demonstrated enhanced biodistribution in folate-expressing tumors. | [143] |
| Paclitaxel liposomes | improvement of loading capacity of hydrophobic drug | Incorporation of triglyceride increased the fluidity and lamellarity of the liposomes thereby resulting in sharp increase in concentration of drug loaded. | [144] |
| DOX liposomes | targeted delivery and cellular uptake | Protonation/deprotonation equilibria to switch the peptide between an “anchored” inactive position and active targeting position within tumor medium. | [145] |
| Irinotecan liposomes | PEG dilemma | PEG-shedding in lower pH achieved by attaching the PEG chain to the lipids via imide bond. | [146] |
| DOX liposomes | endosomal escape | DC-Cholesterol protonation in endosome media adds positive charge to the membrane and facilitates liposome-endosome fusion. | [147] |
| Porphyrin fumed silica–liposome nanocomposite | triggered release | Protonation of the fumed silica surface releases liposomal cargo. | [148] |
| DOX cerasomes | triggered release | Cerasomes were prepared with addition of thermosensitive DPPC and DMPC lipids. | [149] |
| DOX liposome | endosomal escape | Liposomes based on POPC and malachite green derivative carrying a long alkyl chain exhibited fusogenicity following UV irradiation | [150] |
| DOX polymer nanoparticles | endosomal escape | Proton sponge effect by binding protons present in the endo-lysosomes on the tertiary nitrogen atoms in the N,N-dimethylaminoethyl methacrylate units. | [151] |
| Paclitaxel mixed micelles | endosomal escape | Poly(β-amino ester)-mediated endosomal escape through proton-sponge effect. | [152] |
| DOX niosomes | targeted delivery | The glucosamine anchored DOX- loaded targeted niosomes showed the longer circulation in plasma with significantly higher bioavailability | [153] |
| Olanzapine solid lipid nanoparticles | overcoming the BBB | The formulated nanoparticles with olanzapine showed a significant increase in relative bioavailability, i.e., 23-fold in the brain compared to pure olanzapine suspension. | [154] |
| DNA liposome | endosomal escape | Cationic lipids destabilize negatively charged endosomal membranes through ion-pairing mechanism, causing a phase inversion. | [155,156] |
| Polymer nanoparticles | endosomal escape | For polymers (e.g., PEI, chitosan, PAMAM dendrimer) bearing ionogenic groups capable of being protonated at acidic pH additional mechanism is assumed referred to as proton-sponge effect. | [157,158] |
| Amphotericin B chitosan nanoparticles | oral and targeted delivery | An orally active nanomedicine based on an amphiphilic polymer nanoparticle with mucoadhesive properties provides a relative Amphotericin B oral bioavailability of 24.7%. | [159] |
| Telmisartan nanoparticles | instability due to aggregation, poor permeation through cornea | Addition of hydroxypropyl methylcellulose to γ-CD–drug complex | [160] |
| Celecoxib nanoparticles | low drug solubility | Addition of hydroxypropyl methylcellulose to CD–drug complexes | [161] |
| Celecoxib nano- and microparticles | low drug solubility, poor mucoadhesion and cytocompatibility | Addition of hyaluronic acid to randomly methylated β-CD–drug complex | [162] |
| Piroxicam tablets | weak drug release, poor permeation through buccal epithelium | Complexation with CDs was used to provide controlled drug release in vitro, and the additional combination with chitosan increased the permeation of the drug across buccal mucosa. | [163] |
| Simvastatin nanoparticles | low drug solubility, pure absorption and permeation through intestinal mucosa, fast drug release | The drug-loaded nanoparticles suspensions were prepared by ionotropic gelation method using chitosan, sodium tripolyphosphate, β-CD and coated with Eudragit L100. | [164] |
| Eugenol nano- and microparticles | fast drug release | Drug release from electrostatic CD–chitosan aggregates was prolonged due to aggregation ability in contrast to CD–chitosan conjugate. | [165] |
| Itraconazole micelles | low drug solubility, corneal tissue irritation from pluronics | Modification of the pluronics micelles through the incorporation of β-CD and polyethylene oxide. | [166] |
| Econazole nano- and microparticles | instability due to aggregation, low bioavailability | Combination of hydroxypropyl-β-CD with Tween 80 protected the encapsulated drug from aggregation. The suspension in chitosan acidic solution increased the drug bioavailability. | [167] |
| Clotrimazole nanofibers | fast drug release, poor mucoadhesion | Drug-loaded polyvinylpyrrolidone/hydroxypropyl-β-CD fiber was coated with chitosan-cysteine/polyvinyl alcohol. | [168] |
| Camptothecin nanocapsules | low drug loading, fast drug release, intestinal permeability | Addition of chitosan glutamate (PROTASAN™ UP G 113) to amphiphilic CD–drug complex | [169] |
| Timolol maleate compositions | pure permeation through the bovine cornea | Addition of hydroxypropyl-β-CD to bioadhesive polymers | [170] |
| DOX mesoporous silica nanoparticles | poor mucoadhesion | Functionalization of nanoparticles by thiol groups | [171] |
| Docetaxel nanoparticles | low drug loading, fast drug release, intestinal permeability | The anionic emulsion polymerization of isobutylcyanoacrylate was carried out in a solution of methyl-β-CD/drug inclusion complex. | [172] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. https://doi.org/10.3390/ijms21186961
Kashapov R, Gaynanova G, Gabdrakhmanov D, Kuznetsov D, Pavlov R, Petrov K, Zakharova L, Sinyashin O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. International Journal of Molecular Sciences. 2020; 21(18):6961. https://doi.org/10.3390/ijms21186961
Chicago/Turabian StyleKashapov, Ruslan, Gulnara Gaynanova, Dinar Gabdrakhmanov, Denis Kuznetsov, Rais Pavlov, Konstantin Petrov, Lucia Zakharova, and Oleg Sinyashin. 2020. "Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers" International Journal of Molecular Sciences 21, no. 18: 6961. https://doi.org/10.3390/ijms21186961
APA StyleKashapov, R., Gaynanova, G., Gabdrakhmanov, D., Kuznetsov, D., Pavlov, R., Petrov, K., Zakharova, L., & Sinyashin, O. (2020). Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. International Journal of Molecular Sciences, 21(18), 6961. https://doi.org/10.3390/ijms21186961