Interaction between Hemin and Prion Peptides: Binding, Oxidative Reactivity and Aggregation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Binding and Equilibria of Hemin-PrP Complexes
2.1.1. Spectrophotometric Titration of Hemin with PrP106–114
2.1.2. Spectrophotometric Titration of Hemin with PrP95–114
2.1.3. Spectrophotometric Titration of Hemin with PrP84–114
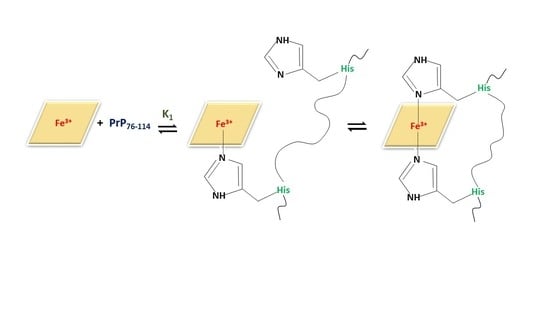
2.1.4. Spectrophotometric Titration of Hemin with PrP76–114
2.1.5. Comparison between Binding Constants of the Complexes between PrP Peptides and Other Neuronal Peptides
2.2. PrP Aggregation Induced by Hemin Studied by Turbidimetry Assay
2.3. Hydrogen Peroxide Activation
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Peptide Synthesis
3.3. Binding Experiments
3.4. Turbidity Measurements
3.5. Kinetic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PrP | Prion protein |
| Aβ | amyloid-β |
| sCJD | sporadic Creutzfeldt–Jakob Disease |
| OR | octa-repeats |
| NMDA | N-methyl-D-aspartate |
| PBS | Phosphate-buffered saline |
| ABTS | 2,2′–azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| TFA | trifluoroacetic acid |
| DMF | dimethylformamide |
| Fmoc | fluorenyl methoxycarbonyl |
| HOBt | N-hydroxybenzotriazole |
| PyBOP | benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate |
| DIEA | N,N-diisopropylethylamine |
| ESI-MS | Electrospray ionization—mass spectrometry |
References
- Frey, P.A.; Reed, G.H. The ubiquity of iron. ACS Chem. Biol. 2012, 7, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, S.; Reynolds, M.F.; Horrigan, F.T.; Heinemann, S.H.; Hoshi, T. Reversible binding of heme to proteins in cellular signal transduction. Acc. Chem. Res. 2006, 39, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, D.; Milward, E.A. Molecular genetic approaches to understanding the roles and regulation of iron in brain health and disease. J. Neurochem. 2010, 113, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.G.; Raven, E.L.; Chernova, T. The regulatory role of heme in neurons. Metallomics 2011, 3, 955–962. [Google Scholar] [CrossRef]
- Chiabrando, D.; Fiorito, V.; Petrillo, S.; Tolosano, E. Unraveling the Role of Heme in Neurodegeneration. Front. Neurosci. 2018, 12, 712. [Google Scholar] [CrossRef]
- Wong, B.X.; Duce, J.A. The iron regulatory capability of the major protein participants in prevalent neurodegenerative disorders. Front. Pharmacol. 2014, 5, 81. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Frey, W.H. A role for heme in Alzheimer’s disease: Heme binds amyloid β and has altered metabolism. Proc. Natl. Acad. Sci. USA 2004, 101, 11153–11158. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Boyle, K. Amyloid-β peptide binds with heme to form a peroxidase: Relationship to the cytopathologies of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 3381–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühl, T.; Imhof, D. Regulatory FeII/III Heme: The Reconstruction of a Molecule’s Biography. ChemBioChem 2014, 15, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, R.; Ashrafi-Kooshk, M.R. Is there correlation between Abeta-heme peroxidase activity and the peptide aggregation state? A literature review combined with hypothesis. Int. J. Biol. Macromol. 2017, 100, 18–36. [Google Scholar] [CrossRef]
- Pramanik, D.; Ghosh, C.; Mukherjee, S.; Dey, S.G. Interaction of amyloid β peptides with redox active heme cofactor: Relevance to Alzheimer’s disease. Coord. Chem. Rev. 2013, 257, 81–92. [Google Scholar] [CrossRef]
- Harrathi, C.; Fernández-Borges, N.; Eraña, H.; Elezgarai, S.R.; Venegas, V.; Charco, J.M.; Castilla, J. Insights into the Bidirectional Properties of the Sheep–Deer Prion Transmission Barrier. Mol. Neurobiol. 2019, 56, 5287–5303. [Google Scholar] [CrossRef] [Green Version]
- Velásquez, C.D.; Kim, C.; Haldiman, T.; Kim, C.; Herbst, A.; Aiken, J.; Safar, J.G.; McKenzie, D. Chronic wasting disease (CWD) prion strains evolve via adaptive diversification of conformers in hosts expressing prion protein polymorphisms. J. Biol. Chem. 2020, 295, 4985–5001. [Google Scholar] [CrossRef] [Green Version]
- Prusiner, S.B. Molecular biology of prion diseases. Science 1991, 252, 1515–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusiner, S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013, 47, 601–623. [Google Scholar] [CrossRef] [Green Version]
- Legname, G. Elucidating the function of the prion protein. PLoS Pathog. 2017, 13, e1006458. [Google Scholar] [CrossRef] [Green Version]
- Naslavsky, N.; Stein, R.; Yanai, A.; Friedlander, G.; Taraboulos, A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J. Biol. Chem. 1997, 272, 6324–6331. [Google Scholar] [CrossRef] [Green Version]
- Wulf, M.A.; Senatore, A.; Aguzzi, A. The biological function of the cellular prion protein: An update. BMC Biol. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasperini, L.; Meneghetti, E.; Pastore, B.; Benetti, F.; Legname, G. Prion protein and copper cooperatively protect neurons by modulating NMDA receptor through S-nitrosylation. Antioxid Redox Signal 2015, 22, 772–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, H.; Tsutsui, S.; Hameed, S.; Kannanayakal, T.J.; Chen, L.; Xia, P.; Engbers, J.D.T.; Lipton, S.A.; Stys, P.K.; Zamponi, G.W. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1737–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanyon, H.F.; Patel, K.; Begum, N.; Viles, J.H. Copper(II) Sequentially Loads onto the N-Terminal Amino Group of the Cellular Prion Protein before the Individual Octarepeats. Biochemistry 2014, 53, 3934–3939. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, C.; Rossetti, G.; Quintanar, L.; Carloni, P. Structural Determinants of the Prion Protein N-Terminus and Its Adducts with Copper Ions. Int. J. Mol. Sci. 2018, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, M.; Walter, E.D.; Newell, D.J.; Jackson, P.J.; Aronoff-Spencer, E.; Peisach, J.; Gerfen, G.J.; Bennett, B.; Antholine, W.E.; Millhauser, G.L. The Octarepeat Domain of the Prion Protein Binds Cu(II) with Three Distinct Coordination Modes at pH 7.4. J. Am. Chem. Soc. 2005, 127, 12647–12656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Natale, G.; Turi, I.; Pappalardo, G.; Sóvágó, I.; Rizzarelli, E. Cross-Talk Between the Octarepeat Domain and the Fifth Binding Site of Prion Protein Driven by the Interaction of Copper(II) with the N-terminus. Chem. A Eur. J. 2015, 21, 4071–4084. [Google Scholar] [CrossRef]
- Younan, N.D.; Sarell, C.J.; Davies, P.; Brown, D.R.; Viles, J.H. The cellular prion protein traps Alzheimer’s Aβ in an oligomeric form and disassembles amyloid fibers. FASEB J. 2013, 27, 1847–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauren, J.; Gimbel, D.A.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-[bgr] oligomers. Nature 2009, 457, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Fluharty, B.R.; Biasini, E.; Stravalaci, M.; Sclip, A.; Diomede, L.; Balducci, C.; La Vitola, P.; Messa, M.; Colombo, L.; Forloni, G.; et al. An N-terminal Fragment of the Prion Protein Binds to Amyloid-β Oligomers and Inhibits Their Neurotoxicity in Vivo. J. Biol. Chem. 2013, 288, 7857–7866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resenberger, U.K.; Winklhofer, K.F.; Tatzelt, J. Cellular prion protein mediates toxic signaling of amyloid beta. Neurodegener. Dis. 2012, 10, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Bacchella, C.; Nicolis, S.; Dell’Acqua, S.; Rizzarelli, E.; Monzani, E.; Casella, L. Membrane Binding Strongly Affecting the Dopamine Reactivity Induced by Copper Prion and Copper/Amyloid-beta (Abeta) Peptides. A Ternary Copper/Abeta/Prion Peptide Complex Stabilized and Solubilized in Sodium Dodecyl Sulfate Micelles. Inorg. Chem. 2020, 59, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; Di Natale, G.; Rizzarelli, E. Copper-assisted interaction between amyloid-β and prion: Ternary metal complexes with Aβ N-terminus and octarepeat. Inorg. Chim. Acta 2018, 472, 93–102. [Google Scholar] [CrossRef]
- Pato, C.; Celier, C.; Rezaei, H.; Grosclaude, J.; Marden, M.C. Heme as an optical probe of a conformational transition of ovine recPrP. Protein Sci. 2004, 13, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Raymond, L.D.; Schoen, B.; Raymond, G.J.; Kett, L.; Moore, R.A.; Johnson, L.M.; Taubner, L.; Speare, J.O.; Onwubiko, H.A.; et al. Hemin Interactions and Alterations of the Subcellular Localization of Prion Protein. J. Biol. Chem. 2007, 282, 36525–36533. [Google Scholar] [CrossRef] [Green Version]
- Soutyrine, A.; Yogasingam, N.; Huang, H.; Mitchell, G. Effects of heme-PrP complex on cell-free conversion and peroxidase-linked immunodetection of prions in blood-based assays. Res. Vet. Sci. 2015, 101, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.K.; Singh, N. Prion Protein-Hemin Interaction Upregulates Hemoglobin Synthesis: Implications for Cerebral Hemorrhage and Sporadic Creutzfeldt-Jakob Disease. J. Alzheimers Dis. 2016, 51, 107–121. [Google Scholar] [CrossRef] [Green Version]
- Priola, S.A.; Raines, A.; Caughey, W.S. Porphyrin and phthalocyanine antiscrapie compounds. Science 2000, 287, 1503–1506. [Google Scholar] [CrossRef] [Green Version]
- Di Natale, G.; Ösz, K.; Nagy, Z.; Sanna, D.; Micera, G.; Pappalardo, G.; Sóvágó, I.; Rizzarell, E. Interaction of Copper(II) with the Prion Peptide Fragment HuPrP(76−114) Encompassing Four Histidyl Residues within and outside the Octarepeat Domain. Inorg. Chem. 2009, 48, 4239–4250. [Google Scholar] [CrossRef]
- de Villiers, K.A.; Kaschula, C.H.; Egan, T.J.; Marques, H.M. Speciation and structure of ferriprotoporphyrin IX in aqueous solution: Spectroscopic and diffusion measurements demonstrate dimerization, but not mu-oxo dimer formation. J. Biol. Inorg. Chem. 2007, 12, 101–117. [Google Scholar] [CrossRef]
- Asher, C.; de Villiers, K.A.; Egan, T.J. Speciation of ferriprotoporphyrin IX in aqueous and mixed aqueous solution is controlled by solvent identity, pH, and salt concentration. Inorg. Chem. 2009, 48, 7994–8003. [Google Scholar] [CrossRef] [PubMed]
- Thiabaud, G.; Pizzocaro, S.; Garcia-Serres, R.; Latour, J.-M.; Monzani, E.; Casella, L. Heme Binding Induces Dimerization and Nitration of Truncated β-Amyloid Peptide Aβ16 Under Oxidative Stress. Angew. Chem. Int. Ed. 2013, 52, 8041–8044. [Google Scholar] [CrossRef] [PubMed]
- Pirota, V.; Monzani, E.; Dell’Acqua, S.; Casella, L. Interactions between heme and tau-derived R1 peptides: Binding and oxidative reactivity. Dalton Trans. 2016, 45, 14343–14351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, J.; Liu, L.; Wang, R.; Lai, X.; Xu, M. Interaction between Amyloid-β Peptide and Heme Probed by Electrochemistry and Atomic Force Microscopy. ACS Chem. Neurosci. 2013, 4, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Atamna, H.; Frey, W.H., 2nd; Ko, N. Human and rodent amyloid-beta peptides differentially bind heme: Relevance to the human susceptibility to Alzheimer’s disease. Arch Biochem. Biophys. 2009, 487, 59–65. [Google Scholar] [CrossRef]
- Yamaguchi, K.I.; Kuwata, K. Formation and properties of amyloid fibrils of prion protein. Biophys. Rev. 2018, 10, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Honda, R.; Kuwata, K. Evidence for a central role of PrP helix 2 in the nucleation of amyloid fibrils. FASEB J. 2018, 32, 3641–3652. [Google Scholar] [CrossRef] [Green Version]
- Jobling, M.F.; Huang, X.; Stewart, L.R.; Barnham, K.J.; Curtain, C.; Volitakis, I.; Perugini, M.; White, A.R.; Cherny, R.A.; Masters, C.L.; et al. Copper and Zinc Binding Modulates the Aggregation and Neurotoxic Properties of the Prion Peptide PrP106−126. Biochemistry 2001, 40, 8073–8084. [Google Scholar] [CrossRef]
- Ghosh, C.; Seal, M.; Mukherjee, S.; Ghosh Dey, S. Alzheimer’s Disease: A Heme–Aβ Perspective. Acc. Chem. Res. 2015, 48, 2556–2564. [Google Scholar] [CrossRef]
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [Green Version]
- Dunford, H.B.; Stillman, J.S. On the function and mechanism of action of peroxidases. Coord. Chem. Rev. 1976, 19, 187–251. [Google Scholar] [CrossRef]
- Monzani, E.; Linati, L.; Casella, L.; De Gioia, L.; Favretto, M.; Gullotti, M.; Chillemi, F. Synthesis, characterization and stereoselective catalytic oxidations of chelated deuterohaemin-glycyl-L-histidine complexes. Inorg. Chim. Acta 1998, 273, 339–345. [Google Scholar] [CrossRef]
- Ryabova, E.S.; Dikiy, A.; Hesslein, A.E.; Bjerrum, M.J.; Ciurli, S.; Nordlander, E. Preparation and reactivity studies of synthetic microperoxidases containing b-type heme. J. Biol. Inorg. Chem. 2004, 9, 385–395. [Google Scholar] [CrossRef]
- Dallacosta, C.; Casella, L.; Monzani, E. Modified microperoxidases exhibit different reactivity towards phenolic substrates. Chembiochem 2004, 5, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Dunford, H.B. Peroxidases in Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 1991; Volume 2, pp. 1–24. [Google Scholar]
- Daglas, M.; Adlard, P.A. The Involvement of Iron in Traumatic Brain Injury and Neurodegenerative Disease. Front. Neurosci. 2018, 12, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’Acqua, S.; Bacchella, C.; Monzani, E.; Nicolis, S.; Di Natale, G.; Rizzarelli, E.; Casella, L. Prion Peptides Are Extremely Sensitive to Copper Induced Oxidative Stress. Inorg. Chem. 2017, 56, 11317–11325. [Google Scholar] [CrossRef] [PubMed]
- Osz, K.; Nagy, Z.; Pappalardo, G.; Di Natale, G.; Sanna, D.; Micera, G.; Rizzarelli, E.; Sovago, I. Copper(II) interaction with prion peptide fragments encompassing histidine residues within and outside the octarepeat domain: Speciation, stability constants and binding details. Chemistry 2007, 13, 7129–7143. [Google Scholar] [CrossRef]
- Edelhoch, H. Spectroscopic Determination of Tryptophan and Tyrosine in Proteins. Biochemistry 1967, 6, 1948–1954. [Google Scholar] [CrossRef]
- Fuhrhop, J.H.; Smith, K.M. Laboratory Methods in Porphyrins and Metalloporphyrins Research; Elsevier: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Fig. P Software Corporation. Version 2.2a; Fig. P Software Corporation: Durham, NC, USA, 1994. [Google Scholar]
- Adams, P.A. The peroxidasic activity of the haem octapeptide microperoxidase-8 (MP-8): The kinetic mechanism of the catalytic reduction of H2O2 by MP-8 using 2,2[prime or minute]-azinobis-(3-ethylbenzothiazoline-6-sulphonate)(ABTS) as reducing substrate. J. Chem. Soc. Perkin Trans. 2 1990, 8, 1407–1414. [Google Scholar] [CrossRef]
- Casella, L.; Monzani, E.; Fantucci, P.; Gullotti, M.; De Gioia, L.; Strini, A.; Chillemi, F. Axial Imidazole Distortion Effects on the Catalytic and Binding Properties of Chelated Deuterohemin Complexes. Inorg. Chem. 1996, 35, 439–444. [Google Scholar] [CrossRef]






| Complex | Log K1 | Log K2 | Log β2 |
|---|---|---|---|
| Hemin-PrP106–114 | // | // | // |
| Hemin-PrP95–114 | 4.80 ± 0.06 | // | // |
| Hemin-PrP84–114 | 6.1 ± 0.3 | 4.72 ± 0.07 e | 10.8 ± 0.4 e |
| Hemin-PrP76–114 | 6.48 ± 0.25 | // | // |
| Hemin-Aβ16 a | 4.80 ± 0.02 | 4.02 | 8.82 ± 0.02 |
| Hemin-AcR1τ b | 6.52 ± 0.02 | <2 | // |
| Hemin-Aβ42 c | 6.86 | 6.46 | 13.32 |
| Hemin-Aβ42 d | 6.85 | // | // |
| Complex | k1 (M−1s−1) | KM (mM) | kcat (s−1) | kcat/KM (M−1s−1) |
|---|---|---|---|---|
| Hemin | 42 ± 4 | 1.90 ± 0.20 | 0.011 ± 0.001 | 6 |
| Hemin-PrP106–114 | 51 ± 5 | 4.70 ± 0.40 * | 0.068 ± 0.002 * | 14 * |
| Hemin-PrP95–114 | 62 ± 1 * | 2.24 ± 0.18 | 0.048 ± 0.012 * | 22 * |
| Hemin-PrP84–114 | 75 ± 4 * | 1.59 ± 0.15 | 0.054 ± 0.001 * | 34 * |
| Hemin-PrP76–114 | 54 ± 1 * | 0.94 ± 0.17 * | 0.056 ± 0.002 * | 60 * |
| Hemin-AcR1τ a | 73 ± 5 | 1.90 ± 0.10 | 0.080 ± 0.002 * | 43 |
| Hemin-Aβ16 a | 43 ± 5 | 0.05 ± 0.05 | 0.070 ± 0.002 | 134 |
| HRP b | 107 | 0.64 | 45.5 | 7.1 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Acqua, S.; Massardi, E.; Monzani, E.; Di Natale, G.; Rizzarelli, E.; Casella, L. Interaction between Hemin and Prion Peptides: Binding, Oxidative Reactivity and Aggregation. Int. J. Mol. Sci. 2020, 21, 7553. https://doi.org/10.3390/ijms21207553
Dell’Acqua S, Massardi E, Monzani E, Di Natale G, Rizzarelli E, Casella L. Interaction between Hemin and Prion Peptides: Binding, Oxidative Reactivity and Aggregation. International Journal of Molecular Sciences. 2020; 21(20):7553. https://doi.org/10.3390/ijms21207553
Chicago/Turabian StyleDell’Acqua, Simone, Elisa Massardi, Enrico Monzani, Giuseppe Di Natale, Enrico Rizzarelli, and Luigi Casella. 2020. "Interaction between Hemin and Prion Peptides: Binding, Oxidative Reactivity and Aggregation" International Journal of Molecular Sciences 21, no. 20: 7553. https://doi.org/10.3390/ijms21207553
APA StyleDell’Acqua, S., Massardi, E., Monzani, E., Di Natale, G., Rizzarelli, E., & Casella, L. (2020). Interaction between Hemin and Prion Peptides: Binding, Oxidative Reactivity and Aggregation. International Journal of Molecular Sciences, 21(20), 7553. https://doi.org/10.3390/ijms21207553