Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells
Abstract
:1. Introduction
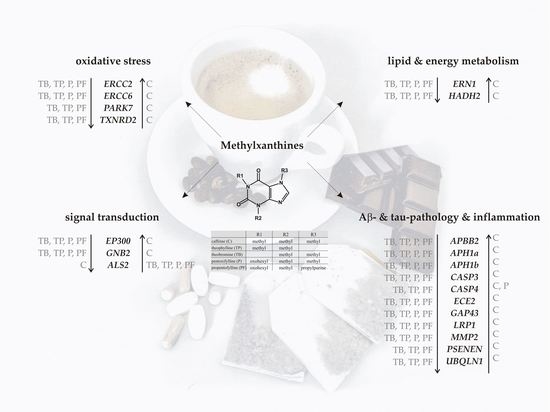
2. Results
2.1. Selection of Stable Housekeeping Genes
2.2. Transcriptional Effects of Caffeine, Theobromine, Theophylline, Pentoxifylline and Propentofylline in Neuronal Cells
2.3. Comparison of Caffeine, Theobromine, Theophylline, Pentoxifylline and Propentofylline in Respect to their Transcription-Regulatory Effects
2.4. Analysis of Cell Type- and Dose-Dependence of the Observerd Methylxanthine-Mediated Effects
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Treatment with Methylxanthines
4.4. Analysis of Gene Expression by RT-PCR Experiments
4.5. Stability Analysis of Housekeeping Genes via NormFinder Algorithm
4.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Franco, R.; Onatibia-Astibia, A.; Martinez-Pinilla, E. Health benefits of methylxanthines in cacao and chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, J.; Mitchell, E.S. More than just caffeine: Psychopharmacology of methylxanthine interactions with plant-derived phytochemicals. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Schienkiewitz, A.; Haftenberger, M.; Mensink, G.B.M. Time trends of non-alcoholic beverage consumption among adults in Germany, 1990–2011. Nutr. J. 2020, 19, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuer, T.; Krems, C.; Moon, K.; Brombach, C.; Hoffmann, I. Food consumption of adults in Germany: Results of the German National Nutrition Survey II based on diet history interviews. Br. J. Nutr. 2015, 113, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Mumford, G.K.; Evans, S.M.; Kaminski, B.J.; Preston, K.L.; Sannerud, C.A.; Silverman, K.; Griffiths, R.R. Discriminative stimulus and subjective effects of theobromine and caffeine in humans. Psychopharmacology 1994, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mumford, G.K.; Benowitz, N.L.; Evans, S.M.; Kaminski, B.J.; Preston, K.L.; Sannerud, C.A.; Silverman, K.; Griffiths, R.R. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur. J. Clin. Pharmacol. 1996, 51, 319–325. [Google Scholar] [CrossRef]
- Anonymous. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Onatibia-Astibia, A.; Martinez-Pinilla, E.; Franco, R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir. Med. 2016, 112, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Onatibia-Astibia, A.; Franco, R.; Martinez-Pinilla, E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017, 61, 1600670. [Google Scholar] [CrossRef]
- Salimi, S.; Fotouhi, A.; Ghoreishi, A.; Derakhshan, M.K.; Khodaie-Ardakani, M.R.; Mohammadi, M.R.; Noorbala, A.A.; Ahmadi-Abhari, S.A.; Hajiazim, M.; Abbasi, S.H.; et al. A placebo controlled study of the propentofylline added to risperidone in chronic schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 726–732. [Google Scholar] [CrossRef]
- Janitschke, D.; Nelke, C.; Lauer, A.A.; Regner, L.; Winkler, J.; Thiel, A.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Effect of caffeine and other methylxanthines on Aβ-homeostasis in SH-SY5Y cells. Biomolecules 2019, 9, 689. [Google Scholar] [CrossRef] [Green Version]
- Neufingerl, N.; Zebregs, Y.E.; Schuring, E.A.; Trautwein, E.A. Effect of cocoa and theobromine consumption on serum HDL-cholesterol concentrations: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 1201–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakuyama, A.; Sadzuka, Y. Effect of methylxanthine derivatives on doxorubicin transport and antitumor activity. Curr. Drug Metab. 2001, 2, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Francis, S.H.; Sekhar, K.R.; Ke, H.; Corbin, J.D. Inhibition of cyclic nucleotide phosphodiesterases by methylxanthines and related compounds. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 93–133. [Google Scholar]
- Aronsen, L.; Orvoll, E.; Lysaa, R.; Ravna, A.W.; Sager, G. Modulation of high affinity ATP-dependent cyclic nucleotide transporters by specific and non-specific cyclic nucleotide phosphodiesterase inhibitors. Eur. J. Pharmacol. 2014, 745, 249–253. [Google Scholar] [CrossRef] [Green Version]
- McPherson, P.S.; Kim, Y.K.; Valdivia, H.; Knudson, C.M.; Takekura, H.; Franzini-Armstrong, C.; Coronado, R.; Campbell, K.P. The brain ryanodine receptor: A caffeine-sensitive calcium release channel. Neuron 1991, 7, 17–25. [Google Scholar] [CrossRef]
- Marangos, P.J.; Paul, S.M.; Parma, A.M.; Goodwin, F.K.; Syapin, P.; Skolnick, P. Purinergic inhibition of diazepam binding to rat brain (in vitro). Life Sci. 1979, 24, 851–857. [Google Scholar] [CrossRef]
- Johnson, I.M.; Prakash, H.; Prathiba, J.; Raghunathan, R.; Malathi, R. Spectral analysis of naturally occurring methylxanthines (theophylline, theobromine and caffeine) binding with DNA. PLoS ONE 2012, 7, e50019. [Google Scholar] [CrossRef] [Green Version]
- Nunnari, G.; Argyris, E.; Fang, J.; Mehlman, K.E.; Pomerantz, R.J.; Daniel, R. Inhibition of HIV-1 replication by caffeine and caffeine-related methylxanthines. Virology 2005, 335, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Lim, S.; Caramori, G.; Cosio, B.; Chung, K.F.; Adcock, I.M.; Barnes, P.J. A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. Proc. Natl. Acad. Sci. USA 2002, 99, 8921–8926. [Google Scholar] [CrossRef] [Green Version]
- Barnes, P.J. Theophylline: New perspectives for an old drug. Am. J. Respir. Crit. Care Med. 2003, 167, 813–818. [Google Scholar] [CrossRef]
- Mitani, T.; Watanabe, S.; Yoshioka, Y.; Katayama, S.; Nakamura, S.; Ashida, H. Theobromine suppresses adipogenesis through enhancement of CCAAT-enhancer-binding protein β degradation by adenosine receptor A1. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.A.; Valero, J. The role of creb signaling in Alzheimer’s disease and other cognitive disorders. Rev. Neurosci. 2011, 22, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Penna, I.; Vella, S.; Gigoni, A.; Russo, C.; Cancedda, R.; Pagano, A. Selection of candidate housekeeping genes for normalization in human postmortem brain samples. Int. J. Mol. Sci. 2011, 12, 5461–5470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 0034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, E.; Levanon, E.Y. Human housekeeping genes, revisited. Trends Genet. 2013, 29, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Riksen, N.P.; Smits, P.; Rongen, G.A. The cardiovascular effects of methylxanthines. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 413–437. [Google Scholar]
- Alsabri, S.G.; Mari, W.O.; Younes, S.; Alsadawi, M.A.; Oroszi, T.L. Kinetic and dynamic description of caffeine. J. Caffeine Adenosine Res. 2018, 8, 3–9. [Google Scholar] [CrossRef]
- Muller, C.E.; Jacobson, K.A. Xanthines as adenosine receptor antagonists. In Methylxanthines; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 200, pp. 151–199. [Google Scholar]
- Liu, R.; Gang, L.; Shen, X.; Xu, H.; Wu, F.; Sheng, L. Binding characteristics and superimposed antioxidant properties of caffeine combined with superoxide dismutase. ACS Omega 2019, 4, 17417–17424. [Google Scholar] [CrossRef]
- Cosio, B.G.; Tsaprouni, L.; Ito, K.; Jazrawi, E.; Adcock, I.M.; Barnes, P.J. Theophylline restores histone deacetylase activity and steroid responses in COPD macrophages. J. Exp. Med. 2004, 200, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-bioactivity relationships of methylxanthines: Trying to make sense of all the promises and the drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef] [Green Version]
- Donoso, P.; O’Neill, S.C.; Dilly, K.W.; Negretti, N.; Eisner, D.A. Comparison of the effects of caffeine and other methylxanthines on [Ca2+]i in rat ventricular myocytes. Br. J. Pharmacol. 1994, 111, 455–458. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.M.; Bhutani, V.K.; Stefano, J.L.; Fox, W.W.; Spitzer, A.R. Changes in pulmonary mechanics following caffeine administration in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 1989, 6, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Endesfelder, S.; Strauss, E.; Scheuer, T.; Schmitz, T.; Buhrer, C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir. Res. 2019, 20, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, M. Cholinesterases, a target of pharmacology and toxicology. Biomed. Pap. 2011, 155, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadsheh, N.; Kussie, P.; Linthicum, D.S. Inhibition of acetylcholinesterase by caffeine, anabasine, methyl pyrrolidine and their derivatives. Toxicol. Lett. 1991, 55, 335–342. [Google Scholar] [CrossRef]
- Blacker, D.; Wilcox, M.A.; Laird, N.M.; Rodes, L.; Horvath, S.M.; Go, R.C.; Perry, R.; Watson, B., Jr.; Bassett, S.S.; McInnis, M.G.; et al. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat. Genet. 1998, 19, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.L.; Geiger, J.D.; Chen, X. Caffeine blocks HIV-1 Tat-induced amyloid beta production and tau phosphorylation. J. Neuroimmune Pharmacol. 2017, 12, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kretzschmar, H.A.; Stowring, L.E.; Westaway, D.; Stubblebine, W.H.; Prusiner, S.B.; Dearmond, S.J. Molecular cloning of a human prion protein cDNA. DNA 1986, 5, 315–324. [Google Scholar] [CrossRef]
- Moon, J.H.; Lee, J.H.; Park, J.Y.; Kim, S.W.; Lee, Y.J.; Kang, S.J.; Seol, J.W.; Ahn, D.C.; Park, S.Y. Caffeine prevents human prion protein-mediated neurotoxicity through the induction of autophagy. Int. J. Mol. Med. 2014, 34, 553–558. [Google Scholar] [CrossRef]
- Byrne, E.M.; Johnson, J.; McRae, A.F.; Nyholt, D.R.; Medland, S.E.; Gehrman, P.R.; Heath, A.C.; Madden, P.A.; Montgomery, G.W.; Chenevix-Trench, G.; et al. A genome-wide association study of caffeine-related sleep disturbance: Confirmation of a role for a common variant in the adenosine receptor. Sleep 2012, 35, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell. Mol. Life Sci. 2004, 61, 857–872. [Google Scholar] [CrossRef]
- Vaugeois, J.M. Signal transduction: Positive feedback from coffee. Nature 2002, 418, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Koh, H.J.; Park, D.C.; Song, B.J.; Huh, T.L.; Park, J.W. Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic. Biol. Med. 2002, 32, 1185–1196. [Google Scholar] [CrossRef]
- Arendash, G.W.; Cao, C. Caffeine and coffee as therapeutics against Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20 (Suppl. 1), S117–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postuma, R.B.; Lang, A.E.; Munhoz, R.P.; Charland, K.; Pelletier, A.; Moscovich, M.; Filla, L.; Zanatta, D.; Romenets, S.R.; Altman, R.; et al. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology 2012, 79, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, J.R.; Dasari, B.; Marwarha, G.; Larson, T.; Chen, X.; Geiger, J.D.; Ghribi, O. Caffeine protects against oxidative stress and Alzheimer’s disease-like pathology in rabbit hippocampus induced by cholesterol-enriched diet. Free Radic. Biol. Med. 2010, 49, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Scarlet, D.; Ertl, R.; Aurich, C.; Steinborn, R. The orthology clause in the next generation sequencing era: Novel reference genes identified by RNA-seq in humans improve normalization of neonatal equine ovary RT-qPCR data. PLoS ONE 2015, 10, e0142122. [Google Scholar] [CrossRef]
- Aithal, M.G.; Rajeswari, N. Validation of housekeeping genes for gene expression analysis in glioblastoma using quantitative real-time polymerase chain reaction. Brain Tumor Res. Treat. 2015, 3, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.; Giacomini, E.; Crinelli, R.; Radici, L.; Carloni, E.; Magnani, M. Dynamic transcription of ubiquitin genes under basal and stressful conditions and new insights into the multiple UBC transcript variants. Gene 2015, 573, 100–109. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Orntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Rohart, F.; Gautier, B.; Singh, A.; Le Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef] [Green Version]







| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| A2M | GCCAACAAGGTGGATTTGAG | AGAGCTCAGCATCAGGCTTC |
| AASS | CTGTGGTGAGAGATGCAGTGA | TGCCCATTGAAAGTGACTGA |
| ABCA1 | TATGAGGGCCAGATCACCTC | TGCTCATCTCAGAGCGAATG |
| ACAT1 | AAATTCATATGGGCAGCTGTG | GCTTCCCATGCTGCTTTACT |
| ACHE | CACTGGTGGGAATGACACAG | ATCTACCACAGGCACGAAGG |
| ACTB | CTTCCTGGGCATGGAGTC | AGCACTGTGTTGGCGTACAG |
| ALS2 | ATACCCTGACACCCAAGCAG | GGTACCTGAGATGGCACTGG |
| APBA1 | CAGGAAGAAGGCTCCTGAAG | GGGTGGTCCATCATTGTCTC |
| APBA3 | CATCTCCTACACAGCCGACA | GATGAGCTGGGCGTCCTC |
| APBB1 | TTTGGAAGGATGAACCCAGT | AAGCTTCTCCTCCTCTTGGG |
| APBB2 | GACCCAGAAGCCAAGTGTTT | GGAAAGTTGCCTGATGCAGT |
| APH1a | CAGCCATTATCCTGCTCCAT | GGAATGTCAGTCCCGATGTC |
| APH1b | GTGTCAGCCCAGACCTTCAT | CAGGCAGAGTTTCAGGCTTC |
| APOA1 | CAGCTAAACCTAAAGCTCCTTGA | CTCAGGCCCTCTGTCTCCTT |
| APOE | GCAGACCGAGTGGCAGAG | CATCAGCGCCCTCAGTTC |
| APPBP1/NAE1 | TCACCAAACAGACTCCATCATT | TTGCCTGAATCTGCAATCATA |
| ATP5B | GCAGGAAAGAATTACCACTACCAAG | TGGTAGCATCCAAATGGGCAA |
| B2M | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT [26] |
| BDNF | TACTTTGGTTGCATGAAGGC | GCCAATGATGTCAAGCCTCT |
| C1orf43 | AGCTCTGGATGCCATTCGTACC | GTGTTTCGCAGATCCAGCAGGT |
| CASP3 | AAGCACTGGAATGACATCTCG | ATCACGCATCAATTCCACAA |
| CASP4 | GGAATGGAGCTGACTTTGACA | CTGACTCCATATCCCTGGCT |
| CAT | ATTCGATCTCACCAAGGTTTG | CTTGGGTCGAAGGCTATCTG |
| CDK5 | TGTGACCAGGACCTGAAGAA | TAGCACATTGCGGCTATGAC |
| CDKL1 | AGGGACCAAAGGGAATAACC | CACCCAACGATGAGTGTTTG |
| CLU | CAGCCCTTCCTTGAGATGAT | CGTCGCCTTCTCGTATGAAT |
| CSTB | CAGGACAAGCACTACGGATACA | CACAGAGAAAGCTCCCTCCA |
| CTSL | CCTGTGAAGAATCAGGGTCAG | GCCCAGAGCAGTCTACCAGA |
| DUOX1 | CTGGACATCCTGGTGGTCTT | ATCCTTGGAAATGAGGCCAT |
| ECE1 | TGATGATCAAGGACGGGAGT | TCTACCATGCACTCGGTCTG |
| ECE2 | ACCTGATCTGGAACCTGGTG | CTCGGCACACAGGACTTCTT |
| EIF4A2 | TGCTCTGCATGGTGACATGGAC | ATCCCGCGAGCCAACAAGTC |
| EMC7 | GTCAGACTGCCCTATCCTCTCC | CATGTCAGGATCACTTGTGTTGAC [50] |
| EP300 | TATGCCAACAGCAGCTCAAC | GGCTTGGACGAGTTTGTGA |
| EPX | GGCGTTGACTGTGAGAGGAC | GGAAGAAAGGGATGCAGTCA |
| ERCC2 | GAGCCCTTTGACGACAGAAC | TGACAGACTGGAAACGCTCA |
| ERCC6 | CAGCGGTTAAGGAGATGGAA | AAACCTTCGTCAAATTCAGCA |
| ERN1 | GTAATCTCTGAGGGCAGCCA | GTCTTCGTGCTTCTCTGGGT |
| GAPDH | ATTCCACCCATGGCAAATTC | GGGATTTCCATTGATGACAAGC |
| GAP43 | AGAGCAGCCAAGCTGAAGAG | CCGGGCACTTTCCTTAGGT |
| GNAO1 | CCAAATACTACCTGGACAGCCT | TGGGTTTCTACGATGCCAGT |
| GNB1 | TTGTGATGCTTCAGCCAAAC | TTTGGAAAGAAGCAAATGGC |
| GNB2 | CCACACTGGGTACCTGTCG | GTCTGCTGGCCTGTCTCAAT |
| GPI | CCAAGTCCAGGGGCGTG | CTTGTTGACCTCTGGCATCACA [50] |
| GPX1 | AGTTTGGGCATCAGGAGAAC | GCACTTCTCGAAGAGCATGA |
| GPX3 | AAGTCGAAGATGGACTGCCA | CCAGCATACTGCTTGAAGGG |
| GPX7 | AACAGGAGCCTGACAGCAAC | AGTCTGGGCCAGGTACTTGAA |
| GSK3a | GGTTCAAGAACCGAGAGCTG | TCGTCTTTCTTCTCGCCACT |
| GSK3b | CGGGATATTAAACCGCAGAA | CGAAACATTGGGTTCTCCTC |
| HADH2/HSD17B10 | CTGTCAACTGTGCAGGCATC | GCCCATGAGATTCACATCAAG |
| HDAC1 | AGCCGGTCATGTCCAAAGTA | TTGGCGTGTCCTTTGATAGTT |
| HMGCR | TCTTCCACGTGCTTGTGACT | CGTGCAAATCTGCTAGTGCT |
| HPRT1 | TGACACTGGCAAAACAATGCA | GGTCCTTTTCACCAGCAAGCT [26] |
| IDH1 | TTTGAAGAAGGTGGTGGTGTT | TCAGATACAAAGGCCAACCC |
| IL1A | GCAGTGAAATTTGACATGGGT | ATCTCCTTCAGCAGCACTGG |
| INSR | TATTGCCTCAAAGGGCTGAA | CTTTGGACAGGAGCAGCATT |
| LPL | GGGCATGTTGACATTTACCC | GCTGGTCCACATCTCCAAGT |
| LRP1 | TGGACTACCAGGATGGGAAG | ATGTCCATGTTGTTGCTGGA |
| LRP6 | CATGGGCCTAAAGGCTACAA | TTCAAAGCCAATAGGGCAAG |
| MAP2 | AATACAGCCCACCTCAGCAG | GGAGGAAGGTCTTGGGAGAG |
| MAPT | AGCCAAGACATCCACACGTT | AGGGTTGGATCAGAGGGTCT |
| MMP2 | ACGACCGCGACAAGAAGTAT | ATTTGTTGCCCAGGAAAGTG |
| MPRIP | ATCTCAGCCATCGAAGCCAT | TGGCTCTTCTCCAGCTCCC |
| NQO1 | AAAGGACCCTTCCGGAGTAA | CTCTGAATTGGCCAGAGAATG |
| NUDT15 | CCAACTCCCTGGAGGTCA | AGCTGCTTCTTCCCAGGTTT |
| PARK7 | TGGTGGTTCTACCAGGAGGT | GTAGGACCTGCACAGATGGC |
| PKP4 | ACAGCATCTGGGACCTTCAC | GGAACTCCGTAAGCCTGTCA |
| PLAT | GAGTGCACCAACTGGAACAG | TAGCACCAGGGCTTTGAGTC |
| PLAU | ACGACATTGCCTTGCTGAAG | GGCAGGCAGATGGTCTGTAT |
| POLR2F | CCCGAAAGATCCCCATCAT | CACCCCCCAGTCTTCATAGC |
| PPP1R15B | AGGTAGTCGGCTTCCAGACA | AGGCCTTCCGTAGAAAGAGG |
| PRDX1 | CAACTGCCAAGTGATTGGTG | CCAAAGGAATGTTCATGGGT |
| PRDX2 | CACCTGGCTTGGATCAACA | GCCGTAATCCTCAGACAAGC |
| PRDX6 | TTGTGAAGAGCCCACAGAAA | AACAAACACCACACGAGCTG |
| PRKAA1 | TGCACACATGAATGCAAAGA | GGCCTGCATACAATCTTCCT |
| PRKAA2 | CCAAATTATGCAGCACCTGA | CATGCTCATCATCAAATGGG |
| PRKCA | ACTTCATGGGATCCCTTTCC | TCCATGTTTCCTTCCTCGTC |
| PRKCD | AGGATGTGGTCCTGATCGAC | AACAGGTGGTCCTTGGTCTG |
| PRKCE | AGCTTTGGCAAGGTCATGTT | CAGTCCACGTCATCATCCTG |
| PRKCG | CAGGAGGAGGGCGAGTATTA | ACCCGCTCATACAATTCCAG |
| PRKCQ | CGAGAAACCATGTTCCACAA | GGTCCCACAGTGTTCACAGA |
| PRKCZ | ACAGACTACGGCATGTGCAA | ACGCTGAACCCGTACTCCT |
| PRNP | AACAAGCCGAGTAAGCCAAA | AAATGTATGATGGGCCTGCT |
| PSENEN | CATCTTCTGGTTCTTCCGAGAG | AGAAGAGGAAGCCCACAGC |
| PSMB4 | CTTGGTGTAGCCTATGAAGCCC | CCAGAACTTCTCGCAGCAGAG [50] |
| RN18S1 | GGAGTATGGTTGCAAAGCTGA | ATCTGTCAATCCTGTCCGTGT [51] |
| RPL13A | CCTGGAGGAGAAGAGGAAAGAGA | TTGAGGACCTCTGTGTATTTGTCAA [26] |
| SDHA | TGGGAACAAGAGGGCATCTG | CCACCACTGCATCAAATTCATG [26] |
| SH2D3C/CHAT | CCAAGGAGATGCAGACCCTA | TGTGGAACCTTTCCAGCAG |
| SNCA | ACCAGTTGGGCAAGAATGAA | CCCTTCCTCAGAAGGCATTT |
| SNCB | CTGGGAGGAGCTGTGTTCTC | CCACTTCCTCTGGCTTCAGAT |
| SNRPD3 | TGCCAGATGTCCAACATCACAGTC | ACATGGGTGCGTTCTTCAGCA |
| SOD1 | CAGCAGGCTGTACCAGTGC | ACATTGCCCAAGTCTCCAAC |
| TBP | CGGAGAGTTCTGGGATTGT | GGTTCGTGGCTCTCTTATC |
| TOP1 | TGAAAGTCCGGCAGAGAGCTG | GCCCACAGTGTCCGCTGTTT |
| TXNIP | TGTGAAGGTGATGAGATTTCCA | GCCATTGGCAAGGTAAGTGT |
| TXNRD2 | GCATGACTGGAGGAAGATGG | AAACCGTGTGCTCGTCAAC |
| UBC | GTGTCTAAGTTTCCCCTTTTAAGG | TTGGGAATGCAACAACTTTATTG [52] |
| UBQLN1 | GCAACCTAGAAAGCATCCCA | TGGATTACCACCAAACTGCTC |
| UCP3 | ATTTCAGGCCAGCATACACC | GGCAAAGTTCCTTTCCACAG |
| UQCRC1 | TGTCAGGAAGCTGTCTCGTG | TCTGGGCGAGGTCTAACAGT |
| UQCRC2 | ATGGCTTTGATTGGACTTGG | TTCACCTCCACGGTAGTTGG |
| XPA | CAACCAGGACCTGTTATGGAA | TGCAGTTATCACAAGTTGGCA |
| YWHAZ | ACTTTTGGTACATTGTGGCTTCAA | CCGCCAGGACAAACCAGTAT [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Seyfried, M.; Grimm, H.S.; Hartmann, T.; Grimm, M.O.W. Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells. Int. J. Mol. Sci. 2020, 21, 9015. https://doi.org/10.3390/ijms21239015
Janitschke D, Lauer AA, Bachmann CM, Seyfried M, Grimm HS, Hartmann T, Grimm MOW. Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells. International Journal of Molecular Sciences. 2020; 21(23):9015. https://doi.org/10.3390/ijms21239015
Chicago/Turabian StyleJanitschke, Daniel, Anna A. Lauer, Cornel M. Bachmann, Martin Seyfried, Heike S. Grimm, Tobias Hartmann, and Marcus O. W. Grimm. 2020. "Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells" International Journal of Molecular Sciences 21, no. 23: 9015. https://doi.org/10.3390/ijms21239015
APA StyleJanitschke, D., Lauer, A. A., Bachmann, C. M., Seyfried, M., Grimm, H. S., Hartmann, T., & Grimm, M. O. W. (2020). Unique Role of Caffeine Compared to Other Methylxanthines (Theobromine, Theophylline, Pentoxifylline, Propentofylline) in Regulation of AD Relevant Genes in Neuroblastoma SH-SY5Y Wild Type Cells. International Journal of Molecular Sciences, 21(23), 9015. https://doi.org/10.3390/ijms21239015