Development and Maintenance of Epidermal Stem Cells in Skin Adnexa
Abstract
:1. Epidermal Stem Cells as Units of Development
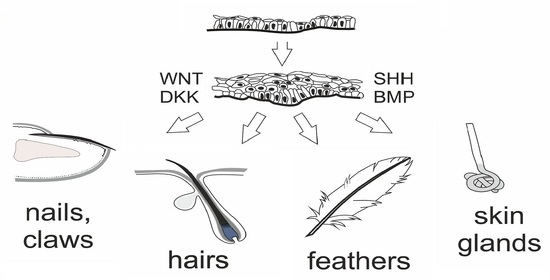
1.1. Development of the Epidermis and Placode Formation
1.2. Development of Hair Follicles
1.3. Hair Pigmentation and Melanocyte SCs
1.4. Development of Sebaceous Gland
1.5. Development of Interfollicular Epidermis
1.6. Development of Other Cutaneous Appendages
2. Epidermal SCs as Units of Tissue Maintenance
2.1. Maintenance of the HF
2.2. Maintenance of McSCs
2.3. Maintenance of SG
2.4. Maintenance of IFE
2.5. Maintenance of Nails
3. Epidermal SC Markers
Author Contributions
Funding
Conflicts of Interest
References
- Byrne, C.; Tainsky, M.; Fuchs, E. Programming gene expression in developing epidermis. Development 1994, 120, 2369–2383. Available online: https://www.ncbi.nlm.nih.gov/pubmed/7525178 (accessed on 18 December 2020). [PubMed]
- Blanpain, C.; Lowry, W.E.; Pasolli, H.A.; Fuchs, E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 2006, 20, 3022–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asare, A.; Levorse, J.; Fuchs, E. Coupling organelle inheritance with mitosis to balance growth and differentiation. Science 2017, 355, eaah4701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, S.J.; Gomez, N.C.; Levorse, J.; Mertz, A.F.; Ge, Y.; Fuchs, E. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature 2019, 569, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Smart, I.H. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br. J. Dermatol. 1970, 82, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.; Asare, A.; Levorse, J.; Ouspenskaia, T.; de la Cruz-Racelis, J.; Schuhmacher, L.N.; Fuchs, E. Progenitors oppositely polarize WNT activators and inhibitors to orchestrate tissue development. Elife 2020, 9, e54304. [Google Scholar] [CrossRef] [Green Version]
- Ouspenskaia, T.; Matos, I.; Mertz, A.F.; Fiore, V.F.; Fuchs, E. WNT-SHH antagonism specifies and expands stem cells prior to niche formation. Cell 2016, 164, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Mok, K.W.; Saxena, N.; Heitman, N.; Grisanti, L.; Srivastava, D.; Muraro, M.J.; Jacob, T.; Sennett, R.; Wang, Z.; Su, Y.; et al. Dermal condensate niche fate specification occurs prior to formation and is placode progenitor dependent. Dev. Cell 2019, 48, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Sick, S.; Reinker, S.; Timmer, J.; Schlake, T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 2006, 314, 1447–1450. [Google Scholar] [CrossRef] [Green Version]
- Schlake, T.; Sick, S. Canonical WNT signalling controls hair follicle spacing. Cell Adh. Migr. 2007, 1, 149–151. [Google Scholar] [CrossRef] [Green Version]
- Dhouailly, D.; Godefroit, P.; Martin, T.; Nonchev, S.; Caraguel, F.; Oftedal, O. Getting to the root of scales, feather and hair: As deep as odontodes? Exp. Dermatol. 2019, 4, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Y.C.; Chuong, C.M. The “tao” of integuments. Science 2016, 354, 1533–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.P.; Polak, L.; Keyes, B.E.; Fuchs, E. Spatiotemporal antagonism in mesenchymal-epithelial signaling in sweat versus hair fate decision. Science 2016, 354, aah6102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, W.M.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012, 26, 1235–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Törnqvist, G.; Sandberg, A.; Hägglund, A.C.; Carlsson, L. Cyclic expression of Lhx2 regulates hair formation. PLoS Genet. 2010, 6, e1000904. [Google Scholar] [CrossRef] [Green Version]
- Frances, D.; Niemann, C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev. Biol. 2012, 363, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.B.; Collins, C.A.; Nascimento, E.; Tan, D.W.; Frye, M.; Itami, S.; Watt, F.M. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 2009, 4, 427–439. [Google Scholar] [CrossRef] [Green Version]
- Kos, R.; Reedy, M.V.; Johnson, R.L.; Erickson, C.A. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development 2001, 128, 1467–1479. Available online: https://pubmed.ncbi.nlm.nih.gov/11262245/ (accessed on 18 December 2020).
- Lee, H.O.; Levorse, J.M.; Shin, M.K. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev. Biol. 2003, 259, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Chuong, C.M.; Lei, M. Regulation of melanocyte stem cells in the pigmentation of skin and its appendages: Biological patterning and therapeutic potentials. Exp. Dermatol. 2019, 28, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Donati, G.; Rognoni, E.; Hiratsuka, T.; Liakath-Ali, K.; Hoste, E.; Kar, G.; Kayikci, M.; Russell, R.; Kretzschmar, K.; Mulder, K.W.; et al. Wounding induces dedifferentiation of epidermal Gata6+ cells and acquisition of stem cell properties. Nat. Cell Biol. 2017, 19, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oulès, B.; Rognoni, E.; Hoste, E.; Goss, G.; Fiehler, R.; Natsuga, K.; Quist, S.; Mentink, R.; Donati, G.; Watt, F.M. Mutant Lef1 controls Gata6 in sebaceous gland development and cancer. EMBO J. 2019, 38, e100526. [Google Scholar] [CrossRef] [PubMed]
- Panteleyev, A.A.; Rosenbach, T.; Paus, R.; Christiano, A.M. The bulge is the source of cellular renewal in the sebaceous gland of mouse skin. Arch. Dermatol. Res. 2000, 292, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, J.G.; Braun, K.M.; Giangreco, A.; van Pelt, C.; Kawamoto, H.; Boyd, R.L.; Willemze, R.; Mullenders, L.H.; Watt, F.M.; de Gruijl, F.R.; et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 2006, 133, 3027–3037. [Google Scholar] [CrossRef] [Green Version]
- Snippert, H.J.; Haegebarth, A.; Kasper, M.; Jaks, V.; van Es, J.H.; Barker, N.; van de Wetering, M.; van den Born, M.; Begthel, H.; Vries, R.G.; et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [Google Scholar] [CrossRef] [Green Version]
- Mack, J.A.; Anand, S.; Maytin, E.V. Proliferation and cornification during development of the mammalian epidermis. Birth Defects Res. C Embryo Today 2005, 75, 314–329. [Google Scholar] [CrossRef]
- Fuchs, E. Skin stem cells: Rising to the surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Dekoninck, S.; Hannezo, E.; Sifrim, A.; Miroshnikova, Y.A.; Aragona, M.; Malfait, M.; Gargouri, S.; de Neunheuser, C.; Dubois, C.; Voet, T.; et al. Defining the design principles of skin epidermis postnatal growth. Cell 2020, 181, 604–620. [Google Scholar] [CrossRef]
- Lu, C.P.; Polak, L.; Rocha, A.S.; Pasolli, H.A.; Chen, S.C.; Sharma, N.; Blanpain, C.; Fuchs, E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 2012, 150, 136–150. [Google Scholar] [CrossRef] [Green Version]
- Headon, D.J. Ectodysplasin signaling in cutaneous appendage development: Dose, duration, and diversity. J. Investig. Dermatol. 2009, 129, 817–819. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.Y.; Yin, M.; Sima, J.; Childress, V.; Michel, M.; Piao, Y.; Schlessinger, D. Involvement of Wnt, Eda and Shh at defined stages of sweat gland development. Development 2014, 141, 3752–3760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haneke, E. Anatomy of the nail unit and the nail biopsy. Semin. Cutan. Med. Surg. 2015, 34, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lehoczky, J.A.; Tabin, C.J. Lgr6 marks nail stem cells and is required for digit tip regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 13249–13254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaks, V.; Barker, N.; Kasper, M.; van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgård, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef] [Green Version]
- Trempus, C.S.; Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Faircloth, R.S.; Reece, J.M.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lyle, S.; Yang, Z.; Cotsarelis, G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Investig. Dermatol. 2003, 121, 963–968. [Google Scholar] [CrossRef] [Green Version]
- Youssef, K.K.; Van Keymeulen, A.; Lapouge, G.; Beck, B.; Michaux, C.; Achouri, Y.; Sotiropoulou, P.A.; Blanpain, C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 2010, 12, 299–305. [Google Scholar] [CrossRef]
- Joost, S.; Zeisel, A.; Jacob, T.; Sun, X.; La Manno, G.; Lönnerberg, P.; Linnarsson, S.; Kasper, M. Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 2016, 3, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Adam, R.C.; Ge, Y.; Hua, Z.L.; Fuchs, E. Epithelial-mesenchymal micro-niches govern stem cell lineage choices. Cell 2017, 169, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Füllgrabe, A.; Joost, S.; Are, A.; Jacob, T.; Sivan, U.; Haegebarth, A.; Linnarsson, S.; Simons, B.D.; Clevers, H.; Toftgård, R.; et al. Dynamics of Lgr6+ progenitor cells in the hair follicle, sebaceous gland, and interfollicular epidermis. Stem Cell Rep. 2015, 5, 843–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales, K.A.U.; Fuchs, E. Skin and its regenerative powers: An alliance between stem cells and their niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Zhang, H.; Duan, E. Epidermal development in mammals: Key regulators, signals from beneath, and stem cells. Int. J. Mol. Sci. 2013, 14, 10869–10895. [Google Scholar] [CrossRef] [PubMed]
- Welle, M.M.; Wiener, D.J. The hair follicle: A comparative review of canine hair follicle anatomy and physiology. Toxicol. Pathol. 2016, 44, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H.; Ferreira, M.; Donati, G.; Marciano, D.K.; Linton, J.M.; Sato, Y.; Hartner, A.; Sekiguchi, K.; Reichardt, L.F.; Watt, F.M. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 2011, 144, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H.; Tsutsui, K.; Morita, R. Multi-tasking epidermal stem cells: Beyond epidermal maintenance. Dev. Growth Differ. 2018, 60, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 2016, 351, aad4395. [Google Scholar] [CrossRef]
- Miyachi, K.; Yamada, T.; Kawagishi-Hotta, M.; Hasebe, Y.; Date, Y.; Hasegawa, S.; Arima, M.; Iwata, Y.; Kobayashi, T.; Numata, S.; et al. Extracellular proteoglycan decorin maintains human hair follicle stem cells. J. Dermatol. 2018, 45, 1403–1410. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Li, K.N.; Jain, P.; He, C.H.; Eun, F.C.; Kang, S.; Tumbar, T. Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. Elife 2019, 8, e45977. [Google Scholar] [CrossRef]
- Di-Poï, N.; Milinkovitch, M.C. The anatomical placode in reptile scale morphogenesis indicates shared ancestry among skin appendages in amniotes. Sci. Adv. 2016, 2, e1600708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.L.; Sun, J.G.; Wu, F.B.; Xi, Y.M. Investigation of characteristics of feather follicle stem cells and their regeneration potential. J. Stem Cells Regen. Med. 2011, 7, 69–74. [Google Scholar] [PubMed]
- Wu, P.; Lai, Y.C.; Widelitz, R.; Chuong, C.M. Comprehensive molecular and cellular studies suggest avian scutate scales are secondarily derived from feathers, and more distant from reptilian scales. Sci. Rep. 2018, 8, 16766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Hou, L. Regulation of melanocyte stem cell behavior by the niche microenvironment. Pigment Cell Melanoma Res. 2018, 5, 556–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 2011, 8, 177–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef]
- Nishimura, E.K. Melanocyte stem cells: A melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011, 24, 401–410. [Google Scholar] [CrossRef]
- Barsh, G.; Gunn, T.; He, L.; Schlossman, S.; Duke-Cohan, J. Biochemical and genetic studies of pigment-type switching. Pigment Cell Res. 2000, 13 (Suppl. 8), 48–53. [Google Scholar] [CrossRef]
- Sharov, A.A.; Fessing, M.; Atoyan, R.; Sharova, T.Y.; Haskell-Luevano, C.; Weiner, L.; Funa, K.; Brissette, J.L.; Gilchrest, B.A.; Botchkarev, V.A. Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Kretzschmar, K.; Cottle, D.L.; Donati, G.; Chiang, M.F.; Quist, S.R.; Gollnick, H.P.; Natsuga, K.; Lin, K.I.; Watt, F.M. BLIMP1 is required for postnatal epidermal homeostasis but does not define a sebaceous gland progenitor under steady-state conditions. Stem Cell Rep. 2014, 3, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, D.; Senoo, M. Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors. J. Investig. Dermatol. 2012, 132, 2461–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Natsuga, K.; Nishie, W.; Kobayashi, Y.; Donati, G.; Suzuki, S.; Fujimura, Y.; Tsukiyama, T.; Ujiie, H.; Shinkuma, S.; et al. Type XVII collagen coordinates proliferation in the interfollicular epidermis. Elife 2017, 6, e26635. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Zhang, T.; Qiao, L.; Du, J.; Li, S.; Zhao, H.; Wang, F.; Huang, Q.; Meng, W.; Zhu, H.; et al. TLR7-expressing cells comprise an interfollicular epidermal stem cell population in murine epidermis. Sci. Rep. 2014, 25, 5831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellheyer, K. Nail stem cells. J. Dtsch. Dermatol. Ges. 2013, 11, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Chou, W.C.; Sun, Q.; Lee, W.; Rabbani, P.; Loomis, C.; Taketo, M.M.; Ito, M. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 2013, 499, 228–232. [Google Scholar] [CrossRef] [Green Version]
- Naveau, A.; Seidel, K.; Klein, O.D. Tooth, hair and claw: Comparing epithelial stem cell niches of ectodermal appendages. Exp. Cell Res. 2014, 325, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Lv, Z.; Nie, M.; Lu, W.; Liu, C.; Tian, Y.; Li, L.; Zhang, G.; Ren, R.; Zhang, Z.; et al. Human nail stem cells are retained but hypofunctional during aging. J. Mol. Histol. 2018, 49, 303–316. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Carmon, K.S.; Lin, Q.; Thomas, A.; Yi, J.; Liu, Q. LGR6 is a high affinity receptor of R-spondins and potentially functions as a tumor suppressor. PLoS ONE 2012, 7, e37137. [Google Scholar] [CrossRef]
- Snippert, H.J.; Clevers, H. Tracking adult stem cells. EMBO Rep. 2011, 12, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.H.; Nguyen, H. Epidermal expression of Lgr6 is dependent on nerve endings and Schwann cells. Exp. Dermatol. 2014, 23, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Barker, N.; Huch, M.; Kujala, P.; van de Wetering, M.; Snippert, H.J.; van Es, J.H.; Sato, T.; Stange, D.E.; Begthel, H.; van den Born, M.; et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010, 6, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, N.; Rookmaaker, M.B.; Kujala, P.; Ng, A.; Leushacke, M.; Snippert, H.; van de Wetering, M.; Tan, S.; Van Es, J.H.; Huch, M.; et al. Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep. 2012, 2, 540–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Visser, K.E.; Ciampricotti, M.; Michalak, E.M.; Tan, D.W.; Speksnijder, E.N.; Hau, C.S.; Clevers, H.; Barker, N.; Jonkers, J. Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland. J. Pathol. 2012, 228, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef]
- Ren, X.; Xia, W.; Xu, P.; Shen, H.; Dai, X.; Liu, M.; Shi, Y.; Ye, X.; Dang, Y. Lgr4 deletion delays the hair cycle and inhibits the activation of hair follicle stem cells. J. Investig. Dermatol. 2020, 140, 1706–1712. [Google Scholar] [CrossRef]
- Page, M.E.; Lombard, P.; Ng, F.; Göttgens, B.; Jensen, K.B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell 2013, 13, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Gur, G.; Rubin, C.; Katz, M.; Amit, I.; Citri, A.; Nilsson, J.; Amariglio, N.; Henriksson, R.; Rechavi, G.; Hedman, H.; et al. LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J. 2004, 23, 3270–3281. [Google Scholar] [CrossRef] [Green Version]
- Laederich, M.B.; Funes-Duran, M.; Yen, L.; Ingalla, E.; Wu, X.; Carraway, K.L., 3rd; Sweeney, C. The leucine-rich repeat protein LRIG1 is a negative regulator of ErbB family receptor tyrosine kinases. J. Biol. Chem. 2004, 279, 47050–47056. [Google Scholar] [CrossRef] [Green Version]
- Waters, J.M.; Richardson, G.D.; Jahoda, C.A. Hair follicle stem cells. Semin. Cell. Dev. Biol. 2007, 18, 245–254. [Google Scholar] [CrossRef]
- Panteleyev, A.A. Functional anatomy of the hair follicle: The Secondary Hair Germ. Exp. Dermatol. 2018, 27, 701–720. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Török, N.; Godbout, M.J.; Lussier, M.; Gaudreau, P.; Royal, A.; Germain, L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: Keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J. Cell Sci. 1996, 109, 1017–1028. [Google Scholar] [PubMed]
- Komine, M.; Freedberg, I.M.; Blumenberg, M. Regulation of epidermal expression of keratin K17 in inflammatory skin diseases. J. Investig. Dermatol. 1996, 107, 569–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Nithya, J. Cytokeratin: A review on current concepts. Int. J. Orofac. Biol. 2018, 2, 6–11. [Google Scholar] [CrossRef]
- Barrandon, Y.; Green, H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc. Natl. Acad. Sci. USA 1985, 82, 5390–5394. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Aoi, N.; Sato, T.; Yamauchi, Y.; Suga, H.; Eto, H.; Kato, H.; Araki, J.; Yoshimura, K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. 2009, 89, 844–856. [Google Scholar] [CrossRef] [Green Version]
- Piwko-Czuchra, A.; Koegel, H.; Meyer, H.; Bauer, M.; Werner, S.; Brakebusch, C.; Fässler, R. Beta1 integrin-mediated adhesion signalling is essential for epidermal progenitor cell expansion. PLoS ONE 2009, 4, e5488. [Google Scholar] [CrossRef] [Green Version]
- Nanba, D. Human keratinocyte stem cells: From cell biology to cell therapy. J. Dermatol. Sci. 2019, 96, 66–72. [Google Scholar] [CrossRef]
- Marconi, A.; Dallaglio, K.; Lotti, R.; Vaschieri, C.; Truzzi, F.; Fantini, F.; Pincelli, C. Survivin identifies keratinocyte stem cells and is downregulated by anti-beta1 integrin during anoikis. Stem Cells 2007, 25, 149–155. [Google Scholar] [CrossRef]
- Kim, S.H.; Sistrunk, C.; Miliani de Marval, P.L.; Rodriguez-Puebla, M.L. Characterization of hair-follicle side population cells in mouse epidermis and skin tumors. Oncol Lett. 2017, 14, 6497–6504. [Google Scholar] [CrossRef]
- Yano, S.; Ito, Y.; Fujimoto, M.; Hamazaki, T.S.; Tamaki, K.; Okochi, H. Characterization and localization of side population cells in mouse skin. Stem Cells 2005, 23, 834–841. [Google Scholar] [CrossRef]
- Rhee, H.; Polak, L.; Fuchs, E. Lhx2 maintains stem cell character in hair follicles. Science 2006, 312, 1946–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrill, B.J.; Gat, U.; DasGupta, R.; Fuchs, E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001, 15, 1688–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Keratin Type | Partner Keratin | Occurrence in Epidermis |
|---|---|---|
| K1 | K10 | differentiated keratinocytes in suprabasal layers [1,82] |
| K2 | K10 | terminally differentiated keratinocytes [1,82] |
| K5 | K14 | proliferative keratinocytes in the basal layer; myoepithelial cells [1,83] |
| K8 | K18 | earliest progenitors lost with skin maturation [83] |
| K9 | K1 | palmar and plantar epidermis [83] |
| K10 | K1 | differentiated keratinocytes in suprabasal layers [1] |
| K14 | K5 | proliferative keratinocytes in the basal layer [1,83] |
| K15 | K5 | HF SCs, restricted to basal cells of stratified epithelia [37,83] |
| K16 | K6 | hyperproliferative/migratory keratinocytes inducible in “activated” epidermis [82,83] |
| K17 | K6 | basal layer; inducible in “activated” epidermis; suprabasal layers in HF; contractile epithelium; myoepithelial cells [82,83] |
| K18 | K8 | earliest progenitors lost with skin maturation; IFE Merkel cells * [81,83] |
| K19 | K7 ** | SCs in HF bulge, basal layer of stratified epithelia; IFE Merkel cells *; eccrine sweat gland secretory cells *** [81,83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokry, J.; Pisal, R. Development and Maintenance of Epidermal Stem Cells in Skin Adnexa. Int. J. Mol. Sci. 2020, 21, 9736. https://doi.org/10.3390/ijms21249736
Mokry J, Pisal R. Development and Maintenance of Epidermal Stem Cells in Skin Adnexa. International Journal of Molecular Sciences. 2020; 21(24):9736. https://doi.org/10.3390/ijms21249736
Chicago/Turabian StyleMokry, Jaroslav, and Rishikaysh Pisal. 2020. "Development and Maintenance of Epidermal Stem Cells in Skin Adnexa" International Journal of Molecular Sciences 21, no. 24: 9736. https://doi.org/10.3390/ijms21249736
APA StyleMokry, J., & Pisal, R. (2020). Development and Maintenance of Epidermal Stem Cells in Skin Adnexa. International Journal of Molecular Sciences, 21(24), 9736. https://doi.org/10.3390/ijms21249736