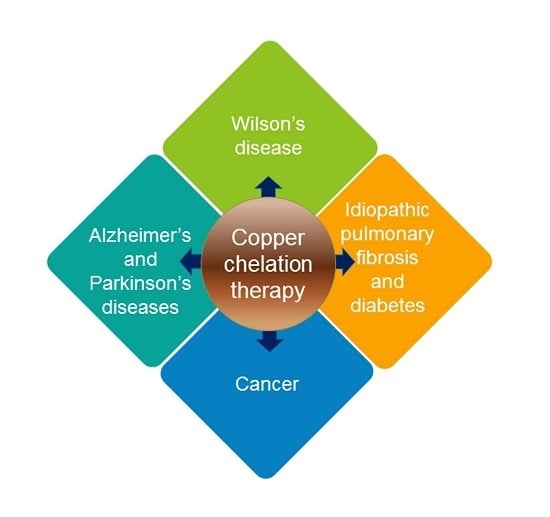
Current Biomedical Use of Copper Chelation Therapy
Abstract
:1. Introduction
2. Clinical Application of Copper Chelation Therapy
2.1. Wilson’s Disease
2.2. Neurological Diseases
2.2.1. Alzheimer’s Disease
2.2.2. Parkinson’s Disease
2.3. Idiopathic Pulmonary Fibrosis
2.4. Diabetes Mellitus
2.5. Cancer
2.5.1. Copper Chelation and Tumor Angiogenesis
2.5.2. Copper Chelation and Inhibition of Tumor Proliferation
2.5.3. Copper Chelation and Tumor Spread
2.5.4. Copper Chelation Combination Therapy Regimens
Copper Chelation and Chemotherapy
Copper Chelation and Radiotherapy
Copper Chelation and Immunotherapy
Copper Chelation and Monoclonal Antibodies Immunotherapy
Copper Chelation and Immune Activation
Copper Chelation and Immune Checkpoint Inhibitors
Copper Chelation and Oncolytic Virotherapy
2.5.5. Copper Depletion and Autophagy Inhibition
| Tumor type | Drug/Intervention | Reference |
|---|---|---|
| Breast cancer | TM | [157] |
| BRAFV600E melanoma | TM | [121,158] |
| BRAFV600E papillary thyroid cancer | TM | [123] |
| BRAFV600E colon cancer | TM | [122] |
| Head and neck | TM | [159,160,161] |
| Endothelial and tumor cells | ATN-224 | [162] |
| Lung cancer and head and neck carcinoma | TM + radiotherapy | [147,163] |
| Esophageal squamous cell carcinoma | TM + cisplatin | [164] |
| Gynecologic cancers | TM + cisplatin | [165] |
| Head and neck carcinoma | TM + OV | [151,152] |
| Head and neck carcinoma | TM + cetuximab | [148] |
| Colorectal cancer | Disulfiram + oxaliplatin | [166] |
| Hepatocellular carcinoma | TETA | [167] |
| Brain tumor | DPA | [168] |
| Mesothelioma | DPA, TETA or TM | [169] |
| Pancreatic duct adenocarcinoma | TM + CQ | [156] |
| Tumor Type | Trial Phase | Patients Enrolled | Drug/Intervention | Reference |
|---|---|---|---|---|
| Metastatic solid tumors including breast, colon, lung, and prostate cancers | I | 18 | TM | [131] |
| Renal cancer | II | 15 | TM | [170] |
| Breast cancer | II | 75 + 40 | TM | [113,171,172] |
| Prostate | II | 19 | TM | [173] |
| Mesothelioma | II | 30 | TM (poa) | [174] |
| Esophageal cancer | II | 69 | TM (poa) | [175] |
| BRAF melanoma | I | wd | Vemurafenib + TETA | NCT02068079 |
| Metastatic colorectal cancer | I | 24 | TM + irinotecan, 5-FU, and IFL | [130] |
| Platinum-resistant epithelial ovarian cancer | I | 5 | TETA plus carboplatin | [137] |
| Head and neck, non-small cell lung and epithelial ovarian | I | 55 | TETA plus carboplatin | [138] |
| Relapse of epithelial ovarian, tubal, and peritoneal cancer | I | 18 | TETA plus carboplatin and PLD | [139] |
| Glioblastoma | II | 40 | DPA | [176] |
| Solid tumors including melanoma and breast, colon, kidney cancers | I | 18 | ATN-224 | [177] |
| Relapsed prostate cancer | II | 47 | ATN-224 | [178] |
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ATN-224 | Choline tetrathiomolybdate |
| ATP7A/7B | Copper-transporting P-type ATPases 7A and 7B |
| BRAF | V-Raf Murine Sarcoma Viral Oncogene Homolog B1 |
| Cp | Ceruloplasmin |
| Cu | Copper |
| CNS | Central Nervous System |
| CTR1 | Copper transport protein 1 |
| EMT | Epithelial mesenchymal transition |
| IPF | Idiopathic pulmonary fibrosis |
| DM | Diabetes mellitus |
| DPA | D-penicillamine |
| LOX | Lysyl Oxidase |
| LOXL | Lysyl Oxidase-Like |
| MAP2K1 | Mitogen-activated protein kinase kinase 1 |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| TETA | Triethylenetetramine |
References
- Blockhuys, S.; Celauro, E.; Hildesjö, C.; Feizi, A.; Stål, O.; Fierro-González, J.C.; Wittung-Stafshede, P. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics 2017, 9, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef]
- Horn, N.; Møller, L.B.; Nurchi, V.M.; Aaseth, J. Chelating principles in Menkes and Wilson diseases: Choosing the right compounds in the right combinations at the right time. J. Inorg. Biochem. 2019, 190, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H.; Maryon, E.B. How mammalian cells acquire copper: An essential but potentially toxic metal. Biophys. J. 2016, 110, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, G.J. Copper in medicine. Curr. Opin. Chem. Biol. 2003, 7, 207–212. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Ding, X.; Xie, H.; Kang, Y.J. The significance of copper chelators in clinical and experimental application. J. Nutr. Biochem. 2011, 22, 301–310. [Google Scholar] [CrossRef]
- Peña, M.M.; Lee, J.; Thiele, D.J. A delicate balance: Homeostatic control of copper uptake and distribution. J. Nutr. 1999, 129, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Weiss, K.H.; Thurik, F.; Gotthardt, D.N.; Schäfer, M.; Teufel, U.; Wiegand, F.; Merle, U.; Ferenci-Foerster, D.; Maieron, A.; Stauber, R.; et al. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin. Gastroenterol. Hepatol. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Lu, J. Triethylenetetramine pharmacology and its clinical applications. Mol. Cancer 2010, 9, 2458–2467. [Google Scholar] [CrossRef] [Green Version]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; de Brouwer, B.; von der Thüsen, J.H.; Wouters, E.F.M. Copper as the most likely pathogenic divergence factor between lung fibrosis and emphysema. Med. Hypotheses 2018, 120, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Taveira-da-Silva, R.; Hilário-Souza, E. Dissecting copper homeostasis in diabetes mellitus. Iubmb Life 2017, 69, 255–262. [Google Scholar] [CrossRef] [Green Version]
- De Luca, A.; Barile, A.; Arciello, M.; Rossi, L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy. J. Trace Elem. Med. Biol. 2019, 55, 204–213. [Google Scholar] [CrossRef]
- Brewer, G.J. Recognition, diagnosis, and management of Wilson’s disease. Proc. Soc. Exp. Biol. Med. 2000, 223, 39–46. [Google Scholar] [CrossRef]
- Bertini, I.; Rosato, A. Menkes disease. Cell Mol. Life Sci. 2008, 65, 89–91. [Google Scholar] [CrossRef]
- Lazoff, S.G.; Rybak, J.J.; Parker, B.R.; Luzzatti, L. Skeletal dysplasia, occipital horns, diarrhea and obstructive uropathy- a new hereditary syndrome. Birth Defects Orig. Artic. Ser. 1975, 11, 71–74. [Google Scholar]
- Vulpe, C.; Levinson, B.; Whitney, S.; Packman, S.; Gitschier, J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993, 3, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Levinson, B.; Vulpe, C.; Whitney, S.; Gitschier, J.; Packman, S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am. J. Hum. Genet. 1995, 56, 570–576. [Google Scholar] [PubMed]
- Vairo, F.P.E.; Chwal, B.C.; Perini, S.; Ferreira, M.A.P.; de Freitas Lopes, A.C.; Saute, J.A.M. A systematic review and evidence-based guideline for diagnosis and treatment of Menkes disease. Mol. Genet. Metab. 2019, 126, 6–13. [Google Scholar] [CrossRef]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Roberts, E.A.; Schilsky, M.L. A practice guideline on Wilson disease. Hepatology 2003, 37, 1475–1492. [Google Scholar] [CrossRef]
- Roberts, E.A.; Schilsky, M.L. Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Mohr, I.; Weiss, K.H. Current anti-copper therapies in management of Wilson disease. Ann. Transl. Med. 2019, 7, S69. [Google Scholar] [CrossRef]
- Walshe, J.M. Penicillamine, a new oral therapy for Wilson’s disease. Am. J. Med. 1956, 21, 487–495. [Google Scholar] [CrossRef]
- Brewer, G.J.; Terry, C.A.; Aisen, A.M.; Hill, G.M. Worsening of neurologic syndrome in patients with Wilson’s disease with initial penicillamine therapy. Arch. Neurol. 1987, 44, 490–493. [Google Scholar] [CrossRef]
- Brewer, G.J.; Yuzbasiyan-Gurkan, V. Wilson disease. Med. (Baltim.) 1992, 71, 139–164. [Google Scholar] [CrossRef]
- Hoogenraad, T.U.; Van Hattum, J.; Van den Hamer, C.J. Management of Wilson’s disease with zinc sulphate. Experience in a series of 27 patients. J. Neurol. Sci. 1987, 77, 137–146. [Google Scholar] [CrossRef]
- Walshe, J.M. Treatment of Wilson’s disease with trientine (triethylene tetramine) dihydrochloride. Lancet 1982, 1, 643–647. [Google Scholar] [CrossRef]
- Menard, M.P.; McCormick, C.C.; Cousins, R.J. Regulation of intestinal metallothionein biosynthesis in rats by dietary zinc. J. Nutr. 1981, 111, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Dick, R.D.; Johnson, V.D.; Brunberg, J.A.; Kluin, K.J.; Fink, J.K. Treatment of Wilson’s disease with zinc: XV long-term follow-up studies. J. Lab. Clin Med. 1998, 132, 264–278. [Google Scholar] [CrossRef]
- Li, W.J.; Chen, C.; You, Z.F.; Yang, R.M.; Wang, X.P. Current drug managements of Wilson’s disease: From West to East. Curr. Neuropharmacol. 2016, 14, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.S.; Zhang, Z.; Wu, J.X.; Li, F.; Xue, B.C.; Yang, R.M. Comparison of long lasting therapeutic effects between succimer and penicillamine on hepatolenticular degeneration. World J. Gastroenterol. 1998, 4, 530–532. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Yuzbasiyan-Gurkin, V.; Tankanow, R.; Young, A.B.; Kluin, K.J. Initial therapy of patients with Wilson’s disease with tetrathiomolybdate. Arch. Neurol. 1991, 48, 42–47. [Google Scholar] [CrossRef]
- Brewer, G.J.; Dick, R.D.; Johnson, V.; Wang, Y.; Yuzbasiyan-Gurkan, V.; Kluin, K.; Fink, J.K.; Aisen, A. Treatment of Wilson’s disease with ammonium tetrathiomolybdate. I. Initial therapy in 17 neurologically affected patients. Arch. Neurol. 1994, 51, 545–554. [Google Scholar] [CrossRef]
- Brewer, G.J.; Johnson, V.; Dick, R.D.; Kluin, K.J.; Fink, J.K.; Brunberg, J.A. Treatment of Wilson disease with ammonium tetrathiomolybdate. II. Initial therapy in 33 neurologically affected patients and follow-up with zinc therapy. Arch. Neurol. 1996, 53, 1017–1025. [Google Scholar] [CrossRef]
- Brewer, G.J.; Askari, F.; Lorincz, M.T.; Carlson, M.; Schilsky, M.; Kluin, K.J.; Hedera, P.; Moretti, P.; Fink, J.K.; Tankanow, R.; et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch. Neurol. 2006, 63, 521–527. [Google Scholar] [CrossRef]
- Weiss, K.H.; Askari, F.K.; Czlonkowska, A.; Ferenci, P.; Bronstein, J.M.; Bega, D.; Ala, A.; Nicholl, D.; Flint, S.; Olsson, L.; et al. Bis-choline tetrathiomolybdate in patients with Wilson’s disease: An open-label, multicentre, phase 2 study. Lancet Gastroenterol. Hepatol. 2017, 2, 869–876. [Google Scholar] [CrossRef]
- Weiss, K.H.; Członkowska, A.; Hedera, P.; Ferenci, P. WTX101–An investigational drug for the treatment of Wilson disease. Expert Opin. Investig. Drugs 2018, 27, 561–567. [Google Scholar] [CrossRef]
- Krishnan, N.; Felice, C.; Rivera, K.; Pappin, D.J.; Tonks, N.K. DPM-1001 decreased copper levels and ameliorated deficits in a mouse model of Wilson’s disease. Genes Dev. 2018, 32, 944–952. [Google Scholar] [CrossRef] [Green Version]
- Lichtmannegger, J.; Leitzinger, C.; Wimmer, R.; Schmitt, S.; Schulz, S.; Kabiri, Y.; Eberhagen, C.; Rieder, T.; Janik, D.; Neff, F.; et al. Methanobactin reverses acute liver failure in a rat model of Wilson disease. J. Clin. Investig. 2016, 126, 2721–2735. [Google Scholar] [CrossRef]
- Tremmel, R.; Uhl, P.; Helm, F.; Wupperfeld, D.; Sauter, M.; Mier, W.; Stremmel, W.; Hofhaus, G.; Fricker, G. Delivery of Copper-chelating Trientine (TETA) to the central nervous system by surface modified liposomes. Int. J. Pharm. 2016, 512, 87–95. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef] [Green Version]
- Hedera, P. Clinical management of Wilson disease. Ann. Transl. Med. 2019, 7, S66. [Google Scholar] [CrossRef]
- Litwin, T.; Dzieżyc, K.; Członkowska, A. Wilson disease-treatment perspectives. Ann. Transl. Med. 2019, 7, S68. [Google Scholar] [CrossRef]
- Schachter, A.S.; Davis, K.L. Alzheimer’s Disease. Curr. Treat. Options Neurol. 2000, 2, 51–60. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Atwood, C.S.; Huang, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I. Role of free radicals and metal ions in the pathogenesis of Alzheimer’s disease. Met. Ions Biol. Syst. 1999, 36, 309–364. [Google Scholar]
- Sensi, S.L.; Granzotto, A.; Siotto, M.; Squitti, R. Copper and zinc dysregulation in Alzheimer’s disease. Trends Pharm. Sci. 2018, 39, 1049–1063. [Google Scholar] [CrossRef]
- Kepp, K.P.; Squitti, R. Copper imbalance in Alzheimer’s disease: Convergence of the chemistry and the clinic. Coord. Chem. Rev. 2019, 397, 168–187. [Google Scholar] [CrossRef]
- Basun, H.; Forssell, L.G.; Wetterberg, L.; Winblad, B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer’s disease. J. Neural. Transm. Park Dis. Dement. Sect. 1991, 3, 231–258. [Google Scholar]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of copper in the onset of Alzheimer’s disease compared to other metals. Front. Aging Neurosci. 2017, 9, 446. [Google Scholar] [CrossRef]
- Squitti, R.; Siotto, M.; Arciello, M.; Rossi, L. Non-ceruloplasmin bound copper and ATP7B gene variants in Alzheimer’s disease. Metallomics 2016, 8, 863–873. [Google Scholar] [CrossRef]
- James, S.A.; Volitakis, I.; Adlard, P.A.; Duce, J.A.; Masters, C.L.; Cherny, R.A.; Bush, A.I. Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic Biol. Med. 2012, 52, 298–302. [Google Scholar] [CrossRef]
- Cherny, R.A.; Atwood, C.S.; Xilinas, M.E.; Gray, D.N.; Jones, W.D.; McLean, C.A.; Barnham, K.J.; Volitakis, I.; Fraser, F.W.; Kim, Y.; et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 2001, 30, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Dedeoglu, A.; Cormier, K.; Payton, S.; Tseitlin, K.A.; Kremsky, J.N.; Lai, L.; Li, X.; Moir, R.D.; Tanzi, R.E.; Bush, A.I.; et al. Preliminary studies of a novel bifunctional metal chelator targeting Alzheimer’s amyloidogenesis. Exp. Gerontol. 2004, 39, 1641–1649. [Google Scholar] [CrossRef]
- Squitti, R.; Rossini, P.M.; Cassetta, E.; Moffa, F.; Pasqualetti, P.; Cortesi, M.; Colloca, A.; Rossi, L.; Finazzi-Agró, A. D-penicillamine reduces serum oxidative stress in Alzheimer’s disease patients. Eur. J. Clin. Investig. 2002, 32, 51–59. [Google Scholar] [CrossRef]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Sampson, E.L.; Jenagaratnam, L.; McShane, R. Metal protein attenuating compounds for the treatment of Alzheimer’s dementia. Cochrane Database Syst. Rev. 2014, CD005380. [Google Scholar] [CrossRef]
- Drew, S.C. The case for abandoning therapeutic chelation of copper ions in Alzheimer’s disease. Front. Neurosci. 2017, 11, 317. [Google Scholar] [CrossRef] [Green Version]
- Squitti, R.; Salustri, C.; Rongioletti, M.; Siotto, M. Commentary: The case for abandoning therapeutic chelation of copper ions in Alzheimer’s disease. Front. Neurol. 2017, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Miotto, M.C.; Rodriguez, E.E.; Valiente-Gabioud, A.A.; Torres-Monserrat, V.; Binolfi, A.; Quintanar, L.; Zweckstetter, M.; Griesinger, C.; Fernández, C.O. Site-specific copper-catalyzed oxidation of α-synuclein: Tightening the link between metal binding and protein oxidative damage in Parkinson’s disease. Inorg. Chem. 2014, 53, 4350–4358. [Google Scholar] [CrossRef]
- Okita, Y.; Rcom-H’cheo-Gauthier, A.N.; Goulding, M.; Chung, R.S.; Faller, P.; Pountney, D.L. Metallothionein, Copper and Alpha-Synuclein in Alpha-Synucleinopathies. Front. Neurosci. 2017, 11, 114. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Willkommen, D.; Skalnaya, A.A.; Danilov, A.B.; Pilipovich, A.A.; Aschner, M.; Skalny, A.V.; Michalke, B.; Skalnaya, M.G. Assessment of copper, iron, zinc and manganese status and speciation in patients with Parkinson’s disease: A pilot study. J. Trace Elem. Med. Biol. 2019, 126423. [Google Scholar] [CrossRef]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s disease: Mechanisms and biochemical processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef]
- Montes, S.; Rivera-Mancia, S.; Diaz-Ruiz, A.; Tristan-Lopez, L.; Rios, C. Copper and copper proteins in Parkinson’s disease. Oxid. Med. Cell Longev. 2014, 2014, 147251. [Google Scholar] [CrossRef] [Green Version]
- Tosato, M.; Di Marco, V. Metal chelation therapy and Parkinson’s disease: A critical review on the thermodynamics of complex formation between relevant metal ions and promising or established drugs. Biomolecules 2019, 9, 269. [Google Scholar] [CrossRef] [Green Version]
- Devos, D.; Moreau, C.; Devedjian, J.C.; Kluza, J.; Petrault, M.; Laloux, C.; Jonneaux, A.; Ryckewaert, G.; Garçon, G.; Rouaix, N.; et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014, 21, 195–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, P.; Kontoghiorghes, G.J. Characterization of the neuroprotective potential of derivatives of the iron chelating drug deferiprone. Neurochem. Res. 2015, 40, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Pashalidis, I.; Kontoghiorghes, G.J. Molecular factors affecting the complex formation between deferiprone (L1) and Cu(II). Possible implications on efficacy and toxicity. Arzneimittelforschung 2001, 51, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Effective inhibition of copper-catalyzed production of hydroxyl radicals by deferiprone. J. Biol. Inorg. Chem. 2019, 24, 331–341. [Google Scholar] [CrossRef]
- Nuñez, M.T.; Chana-Cuevas, P. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel) 2018, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Clarke, D.L.; Murray, L.A.; Crestani, B.; Sleeman, M.A. Is personalised medicine the key to heterogeneity in idiopathic pulmonary fibrosis? Pharm. Ther. 2017, 169, 35–46. [Google Scholar] [CrossRef]
- Kagan, H.M.; Li, W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J. Cell Biochem. 2003, 88, 660–672. [Google Scholar] [CrossRef]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Li, W. LOX/LOXL in pulmonary fibrosis: Potential therapeutic targets. J. Drug Target. 2019, 27, 790–796. [Google Scholar] [CrossRef]
- Brewer, G.J.; Ullenbruch, M.R.; Dick, R.; Olivarez, L.; Phan, S.H. Tetrathiomolybdate therapy protects against bleomycin-induced pulmonary fibrosis in mice. J. Lab. Clin. Med. 2003, 141, 210–216. [Google Scholar] [CrossRef]
- Ovet, H.; Oztay, F. The copper chelator tetrathiomolybdate regressed bleomycin-induced pulmonary fibrosis in mice, by reducing lysyl oxidase expressions. Biol. Trace Elem. Res. 2014, 162, 189–199. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Zhang, F.; Zhu, W.; Wu, J.; Liang, M. Copper in diabetes mellitus: A meta-analysis and systematic review of plasma and serum studies. Biol. Trace Elem. Res. 2017, 177, 53–63. [Google Scholar] [CrossRef]
- Li, P.; Yin, J.; Zhu, Y.; Li, S.; Chen, S.; Sun, T.; Shan, Z.; Wang, J.; Shang, Q.; Li, X.; et al. Association between plasma concentration of copper and gestational diabetes mellitus. Clin. Nutr. 2019, 38, 2922–2927. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, X.K.; Wang, Y.; Cai, L. The role of zinc, copper and iron in the pathogenesis of diabetes and diabetic complications: Therapeutic effects by chelators. Hemoglobin 2008, 32, 135–145. [Google Scholar] [CrossRef]
- Cooper, G.J.; Phillips, A.R.; Choong, S.Y.; Leonard, B.L.; Crossman, D.J.; Brunton, D.H.; Saafi, L.; Dissanayake, A.M.; Cowan, B.R.; Young, A.A.; et al. Regeneration of the heart in diabetes by selective copper chelation. Diabetes 2004, 53, 2501–2508. [Google Scholar] [CrossRef] [Green Version]
- Repiščák, P.; Erhardt, S.; Rena, G.; Paterson, M.J. Biomolecular mode of action of metformin in relation to its copper binding properties. Biochemistry 2014, 53, 787–795. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, K.; Yoshiuchi, K.; Yamasaki, Y.; Shimomura, I.; Matsuoka, T.A.; Matsuhisa, M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 2009, 56, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Coates, R.J.; Weiss, N.S.; Daling, J.R.; Rettmer, R.L.; Warnick, G.R. Cancer risk in relation to serum copper levels. Cancer Res. 1989, 49, 4353–4356. [Google Scholar]
- Margalioth, E.J.; Schenker, J.G.; Chevion, M. Copper and zinc levels in normal and malignant tissues. Cancer 1983, 52, 868–872. [Google Scholar] [CrossRef]
- de Jorge, F.B.; Paiva, L.; Mion, D.; da Nova, R. Biochemical studies on copper, copper oxidase, magnesium, sulfur, calcium and phosphorus in cancer of the larynx. Acta Otolaryngol. 1966, 61, 454–458. [Google Scholar] [CrossRef]
- Shah-Reddy, I.; Khilanani, P.; Bishop, C.R. Serum copper levels in non-Hodgkin’s lymphoma. Cancer 1980, 45, 2156–2159. [Google Scholar] [CrossRef]
- Khadem-Ansari, M.H.; Asoudeh, M.; Gheshlaghi, H.F.K.; Nozari, S.; Zarringol, M.; Maroufi, N.F.; Faridvand, Y. Copper and zinc in stage I multiple myeloma: Relation with ceruloplasmin, lipid peroxidation, and superoxide dismutase activity. Horm. Mol. Biol. Clin. Investig. 2018, 37. [Google Scholar] [CrossRef]
- Kaiafa, G.D.; Saouli, Z.; Diamantidis, M.D.; Kontoninas, Z.; Voulgaridou, V.; Raptaki, M.; Arampatzi, S.; Chatzidimitriou, M.; Perifanis, V. Copper levels in patients with hematological malignancies. Eur. J. Intern. Med. 2012, 23, 738–741. [Google Scholar] [CrossRef]
- Fang, A.P.; Chen, P.Y.; Wang, X.Y.; Liu, Z.Y.; Zhang, D.M.; Luo, Y.; Liao, G.C.; Long, J.A.; Zhong, R.H.; Zhou, Z.G.; et al. Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 144, 2823–2832. [Google Scholar] [CrossRef]
- Zowczak, M.; Iskra, M.; Torliński, L.; Cofta, S. Analysis of serum copper and zinc concentrations in cancer patients. Biol. Trace Elem. Res. 2001, 82, 1–8. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, V.K.; Vaidya, M.P.; Roy, S.K.; Gupta, S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 1993, 52, 172–175. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J. Int. Med. Res. 2018, 46, 4863–4873. [Google Scholar] [CrossRef] [Green Version]
- Turecký, L.; Kalina, P.; Uhlíková, E.; Námerová, S.; Krizko, J. Serum ceruloplasmin and copper levels in patients with primary brain tumors. Klin. Wochenschr. 1984, 62, 187–189. [Google Scholar] [CrossRef]
- Dabek, J.T.; Hyvönen-Dabek, M.; Härkönen, M.; Adlercreutz, H. Evidence for increased non-ceruloplasmin copper in early-stage human breast cancer serum. Nutr. Cancer 1992, 17, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Trovato-Salinaro, A.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis of copper homeostasis genes reveals coordinated upregulation of SLC31A1,SCO1, and COX11 in colorectal cancer. FEBS Open Bio. 2016, 6, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Nagaraja, G.M.; Othman, M.; Fox, B.P.; Alsaber, R.; Pellegrino, C.M.; Zeng, Y.; Khanna, R.; Tamburini, P.; Swaroop, A.; Kandpal, R.P. Gene expression signatures and biomarkers of noninvasive and invasive breast cancer cells: Comprehensive profiles by representational difference analysis, microarrays and proteomics. Oncogene 2006, 25, 2328–2338. [Google Scholar] [CrossRef] [Green Version]
- Goodman, V.L.; Brewer, G.J.; Merajver, S.D. Copper deficiency as an anti-cancer strategy. Endocr. Relat. Cancer 2004, 11, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Ramchandani, D.; Vahdat, L. Copper depletion as a therapeutic strategy in cancer. Met. Ions Life Sci. 2019, 19. [Google Scholar] [CrossRef]
- Garber, K. Cancer’s copper connections. Science 2015, 349, 129. [Google Scholar] [CrossRef]
- Petruzzelli, R.; Polishchuk, R.S. Activity and Trafficking of Copper-Transporting ATPases in Tumor Development and Defense against Platinum-Based Drugs. Cells 2019, 8, 1080. [Google Scholar] [CrossRef] [Green Version]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell Biochem. 1998, 69, 326–335. [Google Scholar] [CrossRef]
- Jain, S.; Cohen, J.; Ward, M.M.; Kornhauser, N.; Chuang, E.; Cigler, T.; Moore, A.; Donovan, D.; Lam, C.; Cobham, M.V.; et al. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann. Oncol. 2013, 24, 1491–1498. [Google Scholar] [CrossRef]
- Soncin, F.; Guitton, J.D.; Cartwright, T.; Badet, J. Interaction of human angiogenin with copper modulates angiogenin binding to endothelial cells. Biochem. Biophys. Res. Commun. 1997, 236, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhang, W.; Kang, Y.J. Copper affects the binding of HIF-1α to the critical motifs of its target genes. Metallomics 2019, 11, 429–438. [Google Scholar] [CrossRef]
- Turski, M.L.; Brady, D.C.; Kim, H.J.; Kim, B.E.; Nose, Y.; Counter, C.M.; Winge, D.R.; Thiele, D.J. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell Biol. 2012, 32, 1284–1295. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S.; Kim, H.W.; Nakagawa, O.; Ozumi, K.; Lessner, S.M.; Aoki, H.; Akram, K.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem. 2008, 283, 9157–9167. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Luo, C.; Shan, C.; You, Q.; Lu, J.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J.L.; Fan, J.; et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 2015, 7, 968–979. [Google Scholar] [CrossRef] [Green Version]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef] [Green Version]
- Brady, D.C.; Crowe, M.S.; Turski, M.L.; Hobbs, G.A.; Yao, X.; Chaikuad, A.; Knapp, S.; Xiao, K.; Campbell, S.L.; Thiele, D.J.; et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Sammons, S.; Brady, D.; Vahdat, L.; Salama, A.K. Copper suppression as cancer therapy: The rationale for copper chelating agents in BRAF V600 mutated melanoma. Melanoma Manag. 2016, 3, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Heffern, M.C.; Su, T.A.; Chang, C.J.; Toietta, G. Effects of copper chelation on BRAF(V600E) positive colon carcinoma cells. Cancers 2019, 11, 659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Casio, M.; Range, D.E.; Sosa, J.A.; Counter, C.M. Copper chelation as targeted therapy in a mouse model of oncogenic BRAF-driven papillary thyroid cancer. Clin. Cancer Res. 2018, 24, 4271–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, R.; Kim, M.J.; Sohn, I.; Kim, S.; Kim, I.; Ryu, J.M.; Choi, H.J.; Kim, J.M.; Lee, S.K.; Yu, J.; et al. Serum trace elements and their associations with breast cancer subgroups in Korean breast cancer patients. Nutrients 2018, 11, 37. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.H.; Hsia, S.M.; Shieh, T.M. Lysyl oxidase and the tumor microenvironment. Int. J. Mol. Sci. 2016, 18, 62. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, G.; Nalvarte, I.; Smirnova, T.; Vecchi, M.; Aceto, N.; Dolemeyer, A.; Frei, A.; Lienhard, S.; Wyckoff, J.; Hess, D.; et al. Memo is a copper-dependent redox protein with an essential role in migration and metastasis. Sci. Signal. 2014, 7, ra56. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Yang, H.; Wu, C.; Dang, X.; Liu, Y. Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition. Sci. Rep. 2015, 5, 12410. [Google Scholar] [CrossRef] [Green Version]
- Blockhuys, S.; Wittung-Stafshede, P. Roles of copper-binding proteins in breast cancer. Int. J. Mol. Sci. 2017, 18, 871. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, M.A.; Jayadev, R.; Miley, G.R.; Blebea, C.A.; Chi, Q.; Ihara, S.; Sherwood, D.R. SPARC promotes cell invasion in vivo by decreasing type IV collagen levels in the basement membrane. Plos Genet. 2016, 12, e1005905. [Google Scholar] [CrossRef] [Green Version]
- Gartner, E.M.; Griffith, K.A.; Pan, Q.; Brewer, G.J.; Henja, G.F.; Merajver, S.D.; Zalupski, M.M. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Investig. New Drugs 2009, 27, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Brewer, G.J.; Dick, R.D.; Grover, D.K.; LeClaire, V.; Tseng, M.; Wicha, M.; Pienta, K.; Redman, B.G.; Jahan, T.; Sondak, V.K.; et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000, 6, 1–10. [Google Scholar] [PubMed]
- Majumder, S.; Chatterjee, S.; Pal, S.; Biswas, J.; Efferth, T.; Choudhuri, S.K. The role of copper in drug-resistant murine and human tumors. Biometals 2009, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Yin, J.Y.; Liu, Z.Q.; Li, X.P. Copper efflux transporters ATP7A and ATP7B: Novel biomarkers for platinum drug resistance and targets for therapy. Iubmb Life 2018, 70, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilari, D.; Guancial, E.; Kim, E.S. Role of copper transporters in platinum resistance. World J. Clin. Oncol. 2016, 7, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Kuo, M.T.; Fu, S.; Savaraj, N.; Chen, H.H. Role of the human high-affinity copper transporter in copper homeostasis regulation and cisplatin sensitivity in cancer chemotherapy. Cancer Res. 2012, 72, 4616–4621. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.H.; Kuo, M.T. Overcoming platinum drug resistance with copper-lowering agents. Anticancer Res. 2013, 33, 4157–4161. [Google Scholar]
- Fu, S.; Naing, A.; Fu, C.; Kuo, M.T.; Kurzrock, R. Overcoming platinum resistance through the use of a copper-lowering agent. Mol. Cancer 2012, 11, 1221–1225. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Hou, M.M.; Wheler, J.; Hong, D.; Naing, A.; Tsimberidou, A.; Janku, F.; Zinner, R.; Piha-Paul, S.; Falchook, G.; et al. Exploratory study of carboplatin plus the copper-lowering agent trientine in patients with advanced malignancies. Investig. New Drugs 2014, 32, 465–472. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuo, M.T.; Liu, Y.S.; Cheng, Y.M.; Wu, P.Y.; Chou, C.Y. A dose escalation study of trientine plus carboplatin and pegylated liposomal doxorubicin in women with a first relapse of epithelial ovarian, tubal, and peritoneal cancer within 12 months after platinum-based chemotherapy. Front. Oncol. 2019, 9, 437. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium compounds as novel adjuvants of chemotherapy drugs-A promising approach to fight cancer drug resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef] [Green Version]
- Kuria, A.; Fang, X.; Li, M.; Han, H.; He, J.; Aaseth, J.O.; Cao, Y. Does dietary intake of selenium protect against cancer? A systematic review and meta-analysis of population-based prospective studies. Crit. Rev. Food Sci. Nutr. 2020, 60, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, M.; Leung, A.W.Y.; Abrams, M.J.; Orvig, C.; Bally, M.B. A Perspective - can copper complexes be developed as a novel class of therapeutics? Dalton Trans. 2017, 46, 10758–10773. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper complexes in cancer therapy. Met. Ions Life Sci. 2018, 18. [Google Scholar] [CrossRef]
- Hedley, D.; Shamas-Din, A.; Chow, S.; Sanfelice, D.; Schuh, A.C.; Brandwein, J.M.; Seftel, M.D.; Gupta, V.; Yee, K.W.; Schimmer, A.D. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leuk Lymphoma 2016, 57, 2437–2440. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Murillo, R.; García-Ramos, J.C.; Ruiz-Azuara, L.; Cheatham, T.E.; Cortés-Guzmán, F. Intercalation processes of copper complexes in DNA. Nucleic Acids Res. 2015, 43, 5364–5376. [Google Scholar] [CrossRef] [Green Version]
- Wachsberger, P.; Burd, R.; Dicker, A.P. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: Exploring mechanisms of interaction. Clin. Cancer Res. 2003, 9, 1957–1971. [Google Scholar]
- Khan, M.K.; Mamou, F.; Schipper, M.J.; May, K.S.; Kwitny, A.; Warnat, A.; Bolton, B.; Nair, B.M.; Kariapper, M.S.; Miller, M.; et al. Combination tetrathiomolybdate and radiation therapy in a mouse model of head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Morisawa, A.; Okui, T.; Shimo, T.; Ibaragi, S.; Okusha, Y.; Ono, M.; Nguyen, T.T.H.; Hassan, N.M.M.; Sasaki, A. Ammonium tetrathiomolybdate enhances the antitumor effects of cetuximab via the suppression of osteoclastogenesis in head and neck squamous carcinoma. Int. J. Oncol. 2018, 52, 989–999. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Qin, J.; Zhou, C.; Wan, G.; Liu, Y.; Zhang, M.; Yang, X.; Zhang, N.; Wang, Y. Multifunctional nanoparticles based on a polymeric copper chelator for combination treatment of metastatic breast cancer. Biomaterials 2019, 195, 86–99. [Google Scholar] [CrossRef]
- Voli, F.; Lerra, L.; Kimpton, K.; Saletta, F.; Shen, S.; Cirillo, G.; Kavallaris, M.; Vittorio, O. Copper homeostasis: A new player in anti-tumor immune response. Cancer Res. 2019, 79. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Pradarelli, J.; Haseley, A.; Wojton, J.; Kaka, A.; Bratasz, A.; Alvarez-Breckenridge, C.A.; Yu, J.G.; Powell, K.; Mazar, A.P.; et al. Copper chelation enhances antitumor efficacy and systemic delivery of oncolytic HSV. Clin. Cancer Res. 2012, 18, 4931–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.Y.; Yu, J.G.; Kaka, A.; Pan, Q.; Kumar, P.; Kumar, B.; Zhang, J.; Mazar, A.; Teknos, T.N.; Kaur, B.; et al. ATN-224 enhances antitumor efficacy of oncolytic herpes virus against both local and metastatic head and neck squamous cell carcinoma. Mol. Oncolytics 2015, 2, 15008. [Google Scholar] [CrossRef] [PubMed]
- Amaravadi, R.K.; Kimmelman, A.C.; Debnath, J. Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 2019, 9, 1167–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 2019, 156, 1173–1189.e1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. bioRxiv 2019, 816587. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, R.; Zhao, Y.; Pan, Y.; Liang, H.; Zhang, J.S.; Tai, S.; Jin, L.; Teng, C.B. Blockage of SLC31A1-dependent copper absorption increases pancreatic cancer cell autophagy to resist cell death. Cell Prolif. 2019, 52, e12568. [Google Scholar] [CrossRef] [Green Version]
- Pan, Q.; Kleer, C.G.; van Golen, K.L.; Irani, J.; Bottema, K.M.; Bias, C.; De Carvalho, M.; Mesri, E.A.; Robins, D.M.; Dick, R.D.; et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002, 62, 4854–4859. [Google Scholar]
- Brady, D.C.; Crowe, M.S.; Greenberg, D.N.; Counter, C.M. Copper chelation inhibits BRAF V600E-driven melanomagenesis and counters resistance to BRAF V600E and MEK1/2 inhibitors. Cancer Res. 2017, 77, 6240–6252. [Google Scholar] [CrossRef] [Green Version]
- Cox, C.; Merajver, S.D.; Yoo, S.; Dick, R.D.; Brewer, G.J.; Lee, J.S.; Teknos, T.N. Inhibition of the growth of squamous cell carcinoma by tetrathiomolybdate-induced copper suppression in a murine model. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 781–785. [Google Scholar] [CrossRef] [Green Version]
- Teknos, T.N.; Islam, M.; Arenberg, D.A.; Pan, Q.; Carskadon, S.L.; Abarbanell, A.M.; Marcus, B.; Paul, S.; Vandenberg, C.D.; Carron, M.; et al. The effect of tetrathiomolybdate on cytokine expression, angiogenesis, and tumor growth in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Hassouneh, B.; Islam, M.; Nagel, T.; Pan, Q.; Merajver, S.D.; Teknos, T.N. Tetrathiomolybdate promotes tumor necrosis and prevents distant metastases by suppressing angiogenesis in head and neck cancer. Mol. Cancer 2007, 6, 1039–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juarez, J.C.; Betancourt, O.; Pirie-Shepherd, S.R.; Guan, X.; Price, M.L.; Shaw, D.E.; Mazar, A.P.; Doñate, F. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin. Cancer Res. 2006, 12, 4974–4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.K.; Miller, M.W.; Taylor, J.; Gill, N.K.; Dick, R.D.; Van Golen, K.; Brewer, G.J.; Merajver, S.D. Radiotherapy and antiangiogenic TM in lung cancer. Neoplasia 2002, 4, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, X.; Fan, T.; Li, Y.; Zhang, K.; Ma, S.; Song, Y.; Xu, J.; Wang, X.; Wang, F.; et al. Enhanced efficacy of combined TM with cisplatin targeting esophageal squamous cell carcinoma via suppressing Stat3 signaling pathway. 2018, 13, 808–815. [Google Scholar] [CrossRef]
- Kim, K.K.; Han, A.; Yano, N.; Ribeiro, J.R.; Lokich, E.; Singh, R.K.; Moore, R.G. Tetrathiomolybdate mediates cisplatin-induced p38 signaling and EGFR degradation and enhances response to cisplatin therapy in gynecologic cancers. Sci. Rep. 2015, 5, 15911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Aparicio, A.; Cornejo, A.; Orue, A.; Rieber, M. Anticancer response to disulfiram may be enhanced by co-treatment with MEK inhibitor or oxaliplatin: Modulation by tetrathiomolybdate, KRAS/BRAF mutations and c-MYC/p53 status. Ecancermedicalscience 2019, 13, 890. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, J.; Yoshiji, H.; Kuriyama, S.; Ikenaka, Y.; Noguchi, R.; Okuda, H.; Tsujinoue, H.; Nakatani, T.; Kishida, H.; Nakae, D.; et al. The copper-chelating agent, trientine, suppresses tumor development and angiogenesis in the murine hepatocellular carcinoma cells. Int. J. Cancer 2001, 94, 768–773. [Google Scholar] [CrossRef]
- Brem, S.S.; Zagzag, D.; Tsanaclis, A.M.; Gately, S.; Elkouby, M.P.; Brien, S.E. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am. J. Pathol. 1990, 137, 1121–1142. [Google Scholar]
- Crowe, A.; Jackaman, C.; Beddoes, K.M.; Ricciardo, B.; Nelson, D.J. Rapid copper acquisition by developing murine mesothelioma: Decreasing bioavailable copper slows tumor growth, normalizes vessels and promotes T cell infiltration. Plos ONE 2013, 8, e73684. [Google Scholar] [CrossRef] [Green Version]
- Redman, B.G.; Esper, P.; Pan, Q.; Dunn, R.L.; Hussain, H.K.; Chenevert, T.; Brewer, G.J.; Merajver, S.D. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin. Cancer Res. 2003, 9, 1666–1672. [Google Scholar]
- Sahota, S.; Willis, A.; Kornhauser, N.; Ward, M.; Cobham, M.; Cigler, T.; Moore, A.; Andreopoulou, E.; Fitzpatrick, V.; Schneider, S.; et al. A phase II study of copper-depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence: Updated results. Cancer Res. 2018, 78, P1-10-02. [Google Scholar] [CrossRef]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the tumor microenvironment: A phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, N.L.; Dunn, R.; Merjaver, S.; Pan, Q.; Pienta, K.J.; Brewer, G.; Smith, D.C. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology 2006, 71, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Pass, H.I.; Brewer, G.J.; Dick, R.; Carbone, M.; Merajver, S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: Final results. Ann. Thorac. Surg. 2008, 86, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Lee, J.S.; Hayman, J.A.; Chang, A.C.; Orringer, M.B.; Pickens, A.; Pan, C.C.; Merajver, S.D.; Urba, S.G. Pre-operative chemoradiation followed by post-operative adjuvant therapy with tetrathiomolybdate, a novel copper chelator, for patients with resectable esophageal cancer. Investig. New Drugs 2013, 31, 435–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brem, S.; Grossman, S.A.; Carson, K.A.; New, P.; Phuphanich, S.; Alavi, J.B.; Mikkelsen, T.; Fisher, J.D. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro. Oncol. 2005, 7, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, S.A.; Adams, A.; Timms, A.; Fisher, N.; Smythe, J.; Watt, S.M.; Joel, S.; Donate, F.; Hayward, C.; Reich, S.; et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 7526–7534. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Zahurak, M.; Beer, T.M.; Ryan, C.J.; Wilding, G.; Mathew, P.; Morris, M.; Callahan, J.A.; Gordon, G.; Reich, S.D.; et al. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naïve prostate cancer. Urol. Oncol. 2013, 31, 581–588. [Google Scholar] [CrossRef] [Green Version]

| Compound Name | Abbreviation | Chemical Formula | Structural Formula |
|---|---|---|---|
| D-penicillamine: (S)-2-amino-3-mercapto-3-methylbutanoic acid | DPA | C5H11NO2S |  |
| Tetrathiomolybdate | TM | MoS4 |  |
| Trientine: triethylenetetramine dihydrochloride | TETA | C6H18N4 |  |
| 5,7-Dichloro-2[(dimethylamino)methyl]quinolin-8-ol | PBT2 | C12H12Cl2N2O |  |
| 2,3-Dimercaptosuccinic acid | DMSA | C4H6O4S2 |  |
| Condition | NCT Number/Reference | Trial Phase | Patients Enrolled | Drug/Intervention | Status |
|---|---|---|---|---|---|
| Wilson’s Disease | NCT02273596 | II | 28 | WTX101 | completed |
| NCT03299829 | n.a. | 50 | TETA | recruiting | |
| NCT01472874 | n.a. | 8 | TETA | completed | |
| NCT01378182 | n.a. | 10 | MSC transplant | completed | |
| Alzheimer’s disease | [60] | n.a. | 34 | DPA | terminated |
| NCT00471211 [61] | n.a. | 78 | PBT2 | completed | |
| Idiopathic pulmonary fibrosis | NCT00189176 | I/II | 23 | TM | completed |
| Diabetes Mellitus | NCT01295073 | II | 0 | TETA | withdrawn |
| NCT01213888 | n.a. | 5 | TETA | terminated |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. https://doi.org/10.3390/ijms21031069
Baldari S, Di Rocco G, Toietta G. Current Biomedical Use of Copper Chelation Therapy. International Journal of Molecular Sciences. 2020; 21(3):1069. https://doi.org/10.3390/ijms21031069
Chicago/Turabian StyleBaldari, Silvia, Giuliana Di Rocco, and Gabriele Toietta. 2020. "Current Biomedical Use of Copper Chelation Therapy" International Journal of Molecular Sciences 21, no. 3: 1069. https://doi.org/10.3390/ijms21031069
APA StyleBaldari, S., Di Rocco, G., & Toietta, G. (2020). Current Biomedical Use of Copper Chelation Therapy. International Journal of Molecular Sciences, 21(3), 1069. https://doi.org/10.3390/ijms21031069