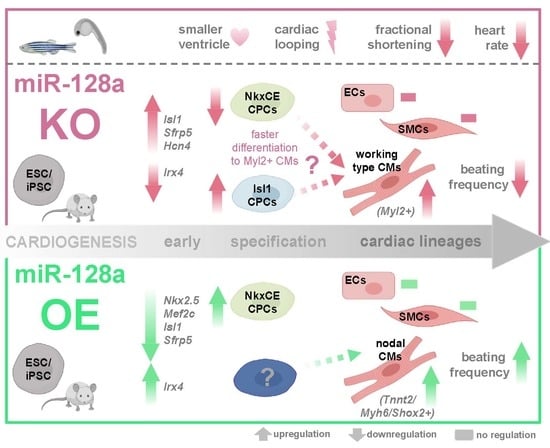
miR-128a Acts as a Regulator in Cardiac Development by Modulating Differentiation of Cardiac Progenitor Cell Populations
Abstract
:1. Introduction
2. Results
2.1. Identification of microRNAs (miRs) Involved in Early Cardiac Development
2.2. miR-128a loss of Function-Induced Cardiac Phenotype in Zebrafish
2.3. Knockdown of miR-128a Promoted Early Cardiogenesis and Favored the Differentiation of NkxCE-GFP CPC Populations In Vitro
2.4. Knockdown of miR-128a Favoured Isl1-Positive CPCs In Vitro
2.5. Overexpression of miR-128a Suppressed Early Cardiogenesis and Retarded Differentiation of NkxCE-GFP-Positive CPC Populations In Vitro
3. Discussion
4. Materials and Methods
4.1. Transgenic Animals
4.2. Human Induced Pluripotent Stem Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bpm | beats per minute |
| CFs | cardiac fibroblasts |
| CMs | cardiomyocytes |
| CPC | cardiac progenitor cells |
| Ctr | control |
| D | day(s) |
| dox | doxycycline |
| E8.5 | embryonic day 8.5 |
| EB | embryoid bodies |
| ECs | endothelial cells |
| ESCs | embryonic stem cells |
| FACS | Fluorescence activated cell sorting (flow cytometry) |
| GFP | green fluorescent protein |
| GO | gene ontology |
| GSEA | gene set enrichment analysis |
| hiPSCs | human induced pluripotent stem cells |
| hpf | hours post fertilization |
| iITG-iPSCs | Isl1-Cre/ROSA26mTmG induced pluripotent stem cells |
| ITG | Isl1-Cre/Rosa26mT/mG |
| KO | knockdown |
| LNA | Locked nucleic acid |
| mG | membrane-tagged GFP |
| miRs | microRNAs |
| MO | morpholino-modified oligonucleotides |
| mRNA | messenger RNA |
| mT | membrane-tagged tdTomato |
| MTT | 3-[4.5-Dimethylthiazol-2-yl]-2.5-di phenyltetrazolium bromide |
| NkxCE | Nkx2.5 cardiac enhancer |
| NkxCE-CPCs | Nkx2.5 cardiac enhancer cardiac progenitor cells |
| NkxCE-GFP | Nkx2.5 cardiac enhancer GFP |
| OE | overexpression |
| P | postnatal day |
| PBMCs | peripheral blood mononuclear cells |
| qRT-PCR | Semiquantitative real time polymerase chain reaction |
| RNA | ribonucleic acid |
| RNAseq | RNA sequencing |
| SAN | sinoatrial node |
| SEM | standard error mean |
| SMCs | smooth muscle cells |
| tet-on | doxycycline-inducible |
| tRFP | Turbo red fluorescent protein |
| TTFs | tail tip fibroblasts |
| UTR | untranslated regions |
| w/o | without |
| wk(s) | week(s) |
References
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–73. [Google Scholar] [CrossRef] [Green Version]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Cordes, K.R.; Srivastava, D. MicroRNA regulation of cardiovascular development. Circ. Res. 2009, 104, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.N.; Muth, A.; Arnold, J.; King, F.W.; Yeh, R.F.; Fish, J.E.; Hsiao, E.C.; Schwartz, R.J.; Conklin, B.R.; Bernstein, H.S.; et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell 2008, 2, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, C.; Han, Z.; Olson, E.N.; Srivastava, D. MicroRNA1 influences cardiac differentiation in Drosophila and regulates Notch signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 18986–18991. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Samal, E.; Srivastava, D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, S.C.; Doppler, S.A.; Dressen, M.; Lahm, H.; Lange, R.; Krane, M. MicroRNAs: Pleiotropic players in congenital heart disease and regeneration. J. Thorac. Dis. 2017, 9, S64–S81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.M.; Fujiwara, Y.; Cibulsky, S.M.; Clapham, D.E.; Lien, C.L.; Schultheiss, T.M.; Orkin, S.H. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 2006, 127, 1137–1150. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Kim, P.J.; Chen, Z.; Lokman, H.; Qiu, L.; Zhang, K.; Rozen, S.G.; Tan, E.K.; Je, H.S.; Zeng, L. MiRNA-128 regulates the proliferation and neurogenesis of neural precursors by targeting PCM1 in the developing cortex. Elife 2016, 5. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, T.; Li, R.; Liu, W.; Chen, Y.; Yan, X.; Gong, Y.; Yin, B.; Liu, W.; Qiang, B.; et al. MicroRNA-128 inhibits glioma cells proliferation by targeting transcription factor E2F3a. J. Mol. Med. 2009, 87, 43–51. [Google Scholar] [CrossRef]
- Witman, N.; Heigwer, J.; Thaler, B.; Lui, W.O.; Morrison, J.I. miR-128 regulates non-myocyte hyperplasia, deposition of extracellular matrix and Islet1 expression during newt cardiac regeneration. Dev. Biol. 2013, 383, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Feng, Y.; Liang, J.; Yu, H.; Wang, C.; Wang, B.; Wang, M.; Jiang, L.; Meng, W.; Cai, W.; et al. Loss of microRNA-128 promotes cardiomyocyte proliferation and heart regeneration. Nat. Commun. 2018, 9, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, R.; Tang, Y.; Zang, W.; Wang, Y.; Li, M.; Du, Y.; Zhao, G.; Xu, Y. MicroRNA-128 regulates the differentiation of rat bone mesenchymal stem cells into neuron-like cells by Wnt signaling. Mol. Cell. Biochem. 2014, 387, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.L.; Wu, C.; Mercer, B.; Webb, R.; Richardson, J.A.; Olson, E.N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 1999, 126, 75–84. [Google Scholar] [PubMed]
- Li, G.; Plonowska, K.; Kuppusamy, R.; Sturzu, A.; Wu, S.M. Identification of cardiovascular lineage descendants at single-cell resolution. Development 2015, 142, 846–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fish, J.E.; Wythe, J.D.; Xiao, T.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D.; Woo, S. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development 2011, 138, 1409–1419. [Google Scholar] [CrossRef] [Green Version]
- Guess, M.G.; Barthel, K.K.; Harrison, B.C.; Leinwand, L.A. miR-30 family microRNAs regulate myogenic differentiation and provide negative feedback on the microRNA pathway. PLoS ONE 2015, 10, e0118229. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, X.; Yu, Z.; Peng, Y.; Zhu, J.; Liu, X.; Li, M.; Han, S.; Zhu, C. Effect of miR-20b on Apoptosis, Differentiation, the BMP Signaling Pathway and Mitochondrial Function in the P19 Cell Model of Cardiac Differentiation In Vitro. PLoS ONE 2015, 10, e0123519. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Liu, Z.; Zhou, L. Roles of miR-17-92 Cluster in Cardiovascular Development and Common Diseases. Biomed. Res. Int. 2017, 2017, 9102909. [Google Scholar] [CrossRef] [Green Version]
- Bem, J.; Grabowska, I.; Daniszewski, M.; Zawada, D.; Czerwinska, A.M.; Bugajski, L.; Piwocka, K.; Fogtman, A.; Ciemerych, M.A. Transient MicroRNA Expression Enhances Myogenic Potential of Mouse Embryonic Stem Cells. Stem Cells 2018, 36, 655–670. [Google Scholar] [CrossRef] [Green Version]
- Razak, S.R.; Ueno, K.; Takayama, N.; Nariai, N.; Nagasaki, M.; Saito, R.; Koso, H.; Lai, C.Y.; Murakami, M.; Tsuji, K.; et al. Profiling of microRNA in human and mouse ES and iPS cells reveals overlapping but distinct microRNA expression patterns. PLoS ONE 2013, 8, e73532. [Google Scholar] [CrossRef] [Green Version]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, L.; Scott, I.C.; Jungblut, B.; Stainier, D.Y. A mutation in zebrafish hmgcr1b reveals a role for isoprenoids in vertebrate heart-tube formation. Curr. Biol. 2007, 17, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Ketley, A.; Warren, A.; Holmes, E.; Gering, M.; Aboobaker, A.A.; Brook, J.D. The miR-30 microRNA family targets smoothened to regulate hedgehog signalling in zebrafish early muscle development. Plos One 2013, 8, e65170. [Google Scholar] [CrossRef]
- Wang, J.; Cao, N.; Yuan, M.; Cui, H.; Tang, Y.; Qin, L.; Huang, X.; Shen, N.; Yang, H.T. MicroRNA-125b/Lin28 pathway contributes to the mesendodermal fate decision of embryonic stem cells. Stem Cells Dev. 2012, 21, 1524–1537. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4. [Google Scholar] [CrossRef]
- Wong, N.; Wang, X. miRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, M.; Sakaguchi, A.; Kamata, R.; Nagao, M.; Kikuchi, Y.; Evans, S.M.; Yoshizumi, M.; Shimono, A.; Saga, Y.; Kokubo, H. Sfrp5 identifies murine cardiac progenitors for all myocardial structures except for the right ventricle. Nat. Commun. 2017, 8, 14664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moretti, A.; Caron, L.; Nakano, A.; Lam, J.T.; Bernshausen, A.; Chen, Y.; Qyang, Y.; Bu, L.; Sasaki, M.; Martin-Puig, S.; et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 2006, 127, 1151–1165. [Google Scholar] [CrossRef] [Green Version]
- Spater, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell Biol. 2013, 15, 1098–1106. [Google Scholar] [CrossRef]
- Nelson, D.O.; Lalit, P.A.; Biermann, M.; Markandeya, Y.S.; Capes, D.L.; Addesso, L.; Patel, G.; Han, T.; John, M.C.; Powers, P.A.; et al. Irx4 Marks a Multipotent, Ventricular-Specific Progenitor Cell. Stem Cells 2016, 34, 2875–2888. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.D.; Tian, Y.; Morrisey, E.E. Wnt signaling: An essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 2008, 135, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Sklepkiewicz, P.; Shiomi, T.; Kaur, R.; Sun, J.; Kwon, S.; Mercer, B.; Bodine, P.; Schermuly, R.T.; George, I.; Schulze, P.C.; et al. Loss of secreted frizzled-related protein-1 leads to deterioration of cardiac function in mice and plays a role in human cardiomyopathy. Circ. Heart Fail. 2015, 8, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Loeys, B.L.; Chen, J.; Neptune, E.R.; Judge, D.P.; Podowski, M.; Holm, T.; Meyers, J.; Leitch, C.C.; Katsanis, N.; Sharifi, N.; et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005, 37, 275–281. [Google Scholar] [CrossRef]
- Nothjunge, S.; Nuhrenberg, T.G.; Gruning, B.A.; Doppler, S.A.; Preissl, S.; Schwaderer, M.; Rommel, C.; Krane, M.; Hein, L.; Gilsbach, R. DNA methylation signatures follow preformed chromatin compartments in cardiac myocytes. Nat. Commun. 2017, 8, 1667. [Google Scholar] [CrossRef] [Green Version]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, C.L.; Lin, L.; Qyang, Y.; Chung, C.; Monteiro, R.M.; Mummery, C.L.; Fishman, G.I.; Cogen, A.; Evans, S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development 2006, 133, 1575–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, C.A.; Stadtfeld, M.; Murphy, G.J.; Hochedlinger, K.; Kotton, D.N.; Mostoslavsky, G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells 2009, 27, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Ficca, M.L.; Meyer, R.G.; Kaiser, H.; Brack, A.R.; Kandolf, R.; Kupper, J.H. Comparative analysis of inducible expression systems in transient transfection studies. Anal. Biochem. 2004, 334, 9–19. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Hayakawa, T. Characteristics of adenovirus-mediated tetracycline-controllable expression system. Biochim. Biophys. Acta 2001, 1568, 21–29. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, H.E.; Lee, K.H.; Han, W.; Yi, M.J.; Jeong, J.; Oh, B.H. Two-promoter vector is highly efficient for overproduction of protein complexes. Protein Sci. 2004, 13, 1698–1703. [Google Scholar] [CrossRef] [Green Version]
- Ahler, E.; Sullivan, W.J.; Cass, A.; Braas, D.; York, A.G.; Bensinger, S.J.; Graeber, T.G.; Christofk, H.R. Doxycycline alters metabolism and proliferation of human cell lines. PLoS ONE 2013, 8, e64561. [Google Scholar] [CrossRef]
- Herbst, F.; Ball, C.R.; Tuorto, F.; Nowrouzi, A.; Wang, W.; Zavidij, O.; Dieter, S.M.; Fessler, S.; van der Hoeven, F.; Kloz, U.; et al. Extensive methylation of promoter sequences silences lentiviral transgene expression during stem cell differentiation in vivo. Mol. Ther. 2012, 20, 1014–1021. [Google Scholar] [CrossRef] [Green Version]
- Bober, E.; Lyons, G.E.; Braun, T.; Cossu, G.; Buckingham, M.; Arnold, H.H. The muscle regulatory gene, Myf-6, has a biphasic pattern of expression during early mouse development. J. Cell Biol. 1991, 113, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Doppler, S.A.; Werner, A.; Barz, M.; Lahm, H.; Deutsch, M.A.; Dressen, M.; Schiemann, M.; Voss, B.; Gregoire, S.; Kuppusamy, R.; et al. Myeloid zinc finger 1 (Mzf1) differentially modulates murine cardiogenesis by interacting with an Nkx2.5 cardiac enhancer. PLoS ONE 2014, 9, e113775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.; Bodmer, R.; Abmayr, S.M.; McDermott, J.C.; Spoerel, N.A. D-mef2: A Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 7520–7524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, C.T.; Lu, Q.; Wang, Y.; Chen, J.N. Zebrafish as a model for cardiovascular development and disease. Drug Discov. Today Dis. Models 2008, 5, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stainier, D.Y. Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef]
- Grant, M.G.; Patterson, V.L.; Grimes, D.T.; Burdine, R.D. Modeling Syndromic Congenital Heart Defects in Zebrafish. Curr. Top. Dev. Biol. 2017, 124, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Andres-Delgado, L.; Mercader, N. Interplay between cardiac function and heart development. Biochim. Biophys. Acta 2016, 1863, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saga, Y. Genetic rescue of segmentation defect in MesP2-deficient mice by MesP1 gene replacement. Mech. Dev. 1998, 75, 53–66. [Google Scholar] [CrossRef]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Holper, S.; Kruger, M.; Stainier, D.Y. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Dorn, T.; Goedel, A.; Lam, J.T.; Haas, J.; Tian, Q.; Herrmann, F.; Bundschu, K.; Dobreva, G.; Schiemann, M.; Dirschinger, R.; et al. Direct nkx2-5 transcriptional repression of isl1 controls cardiomyocyte subtype identity. Stem Cells 2015, 33, 1113–1129. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.J.; Quaranta, R.; Piccini, I.; Fell, J.; Rao, J.; Ropke, A.; Seebohm, G.; Greber, B. Cardiogenic programming of human pluripotent stem cells by dose-controlled activation of EOMES. Nat. Commun. 2018, 9, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Weerd, J.H.; Christoffels, V.M. The formation and function of the cardiac conduction system. Development 2016, 143, 197–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.Z.; Xie, Y.; Moyes, K.W.; Gold, J.D.; Askari, B.; Laflamme, M.A. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ. Res. 2010, 107, 776–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Liang, X.; Cheedipudi, S.; Cordero, J.; Jiang, X.; Zhang, Q.; Caputo, L.; Gunther, S.; Kuenne, C.; Ren, Y.; et al. Pioneering function of Isl1 in the epigenetic control of cardiomyocyte cell fate. Cell Res. 2019, 29, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoelscher, S.C.; Stich, T.; Diehm, A.; Lahm, H.; Dreßen, M.; Zhang, Z.; Neb, I.; Aherrahrou, Z.; Erdmann, J.; Schunkert, H.; et al. miR-128a Acts as a Regulator in Cardiac Development by Modulating Differentiation of Cardiac Progenitor Cell Populations. Int. J. Mol. Sci. 2020, 21, 1158. https://doi.org/10.3390/ijms21031158
Hoelscher SC, Stich T, Diehm A, Lahm H, Dreßen M, Zhang Z, Neb I, Aherrahrou Z, Erdmann J, Schunkert H, et al. miR-128a Acts as a Regulator in Cardiac Development by Modulating Differentiation of Cardiac Progenitor Cell Populations. International Journal of Molecular Sciences. 2020; 21(3):1158. https://doi.org/10.3390/ijms21031158
Chicago/Turabian StyleHoelscher, Sarah C., Theresia Stich, Anne Diehm, Harald Lahm, Martina Dreßen, Zhong Zhang, Irina Neb, Zouhair Aherrahrou, Jeanette Erdmann, Heribert Schunkert, and et al. 2020. "miR-128a Acts as a Regulator in Cardiac Development by Modulating Differentiation of Cardiac Progenitor Cell Populations" International Journal of Molecular Sciences 21, no. 3: 1158. https://doi.org/10.3390/ijms21031158
APA StyleHoelscher, S. C., Stich, T., Diehm, A., Lahm, H., Dreßen, M., Zhang, Z., Neb, I., Aherrahrou, Z., Erdmann, J., Schunkert, H., Santamaria, G., Cuda, G., Gilsbach, R., Hein, L., Lange, R., Hassel, D., Krane, M., & Doppler, S. A. (2020). miR-128a Acts as a Regulator in Cardiac Development by Modulating Differentiation of Cardiac Progenitor Cell Populations. International Journal of Molecular Sciences, 21(3), 1158. https://doi.org/10.3390/ijms21031158