Artocarpus lakoocha Extract Inhibits LPS-Induced Inflammatory Response in RAW 264.7 Macrophage Cells
Abstract
:1. Introduction
2. Results
2.1. Chemical Profile of Oxyresveratrol in Artocarpus Lakoocha Roxb. (AL) Extract
2.2. Effects of AL on the Viability of RAW 264.7 Cells
2.3. Effects of AL on Inflammatory Cytokine Production in Lipopolysaccharide (LPS)-Induced RAW 264.7 Cells
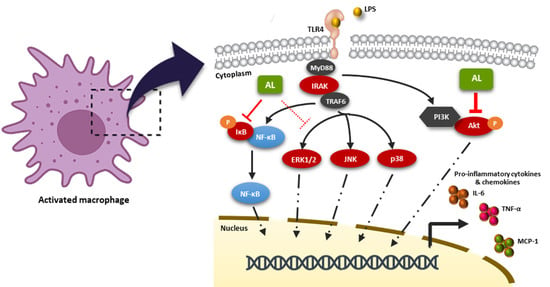
2.4. Effects of AL on Suppressing Nuclear Factor-kappa B (NF-κB) Activation in LPS-Induced RAW 246.7 Cells
2.5. AL Inhibits LPS-Induced Mitogen-activated Protein Kinase (MAPK) and Akt Signaling Activation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction of Artocarpus Lakoocha (AL) Heartwoods
4.2. High-Performance Liquid Chromatograph Analysis (HPLC)
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Nitric Oxide (NO) Production Assay
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Western Blot Analysis
4.8. Immunofluorescence Study
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | Artocarpus lakoocha |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| HPLC | High-performance liquid chromatograph |
| IL-6 | Interleukin 6 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NF-κB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| TNF-α | Tumor necrosis factor-alpha |
| TLR4 | Toll-like receptor 4 |
References
- A current view on inflammation. Nat. Immunol. 2017, 18, 825. [CrossRef] [PubMed] [Green Version]
- Hattori, Y.S.; Kasai, K. Lipopolysaccharide activates Akt in vascular smooth muscle cells resulting in induction of inducible nitric oxide synthase through nuclear factor-kappa B activation. Eur. J. Pharm. 2003, 481, 153–158. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Savva, A.; Roger, T. Targeting toll-like receptors: Promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases. Front. Immunol. 2013, 4, 387. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Immune Receptors and Signal Transduction. Cell. Mol. Immunol. 2015, 8, 137–169. [Google Scholar]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Palanuvej, C.; Issaravanich, S.; Tunsaringkarn, T.; Rungsiyothin, A.; Vipunngeun, N.; Ruangrungsi, N.; Likhitwitayawuid, K. Pharmacognostic study of Artocarpus lakoocha heartwood. J. Health Res. 2007, 4, 257–262. [Google Scholar]
- Likhitwitayawuid, K.; Sornsute, A.; Sritularak, B.; Ploypradith, P. Chemical transformations of oxyresveratrol (trans-2,4,3′,5′-tetrahydroxystilbene) into a potent tyrosinase inhibitor and a strong cytotoxic agent. Bioorg. Med. Chem. Lett. 2006, 16, 5650–5653. [Google Scholar] [CrossRef]
- Hu, C.; Chen, Z.; Yao, R.; Xu, G. Inhibition of protein kinase C by stilbene derivatives from Monus alba L. Nat. Prod. Res. Dev. 1996, 8, 13–16. [Google Scholar]
- Mazimba, O.R.; Majinda, D. Motlhanka. Antioxidant and antibacterial constituents from Morus nigra. Afr. J. Pharm. Pharm. 2011, 5, 751–754. [Google Scholar] [CrossRef] [Green Version]
- Chuanasa, T.; Phromjai, J.; Lipipun, V.; Likhitwitayawuid, K.; Suzuki, M.; Pramyothin, P.; Hattori, M. Mechanism of action and therapeutic efficacy on cutaneous HSV-1 infection in mice. Antivir. Res. 2008, 80, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Sritularak, B.; Benchanak, K.; Lipipun, V.; Mathew, J.; Schinazi, R.F. Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. Nat. Prod. Res. 2005, 19, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Supudompo, B.; Sritularak, B.; Lipipun, V.; Rapp, K. Phenolics with Anti-HSV and Anti-HIV Activities from Artocarpus gomezianus, Mallotus pallidus, and Triphasia trifolia. Pharm. Biol. 2008, 43, 651–657. [Google Scholar] [CrossRef]
- Lipipun, V.; Sasivimolphan, P.; Yoshida, Y.; Daikoku, T.; Sritularak, B.; Ritthidej, G.; Likhitwitayawuid, K.; Pramyothin, P.; Hattori, M.; Shiraki, K. Topical cream-based oxyresveratrol in the treatment of cutaneous HSV-1 infection in mice. Antivir. Res. 2011, 91, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Sasivimolphan, P.; Lipipun, V.; Ritthidej, G.; Chitphet, K.; Yoshida, Y.; Daikoku, T.; Sritularak, B.; Likhitwitayawuid, K.; Pramyothin, P.; Hattori, M.; et al. Microemulsion-based oxyresveratrol for topical treatment of herpes simplex virus (HSV) infection: Physicochemical properties and efficacy in cutaneous HSV-1 infection in mice. Aaps Pharmscitech 2012, 13, 1266–1275. [Google Scholar] [CrossRef] [Green Version]
- Povichit, N.; Phrutivorapongkul, A.; Suttajit, M.; Leelapornpisid, P. Antiglycation and antioxidant activities of oxyresveratrol extracted from the heartwood of Artocarpus lakoocha Roxb. Maejo Int. Sci. Technol. 2010, 4, 454–461. [Google Scholar]
- Chen, Y.-C.; Tien, Y.-J.; Chen, C.-H.; Beltran, F.N.; Amor, E.C.; Wang, R.-J.; Wu, D.-J.; Mettling, C.; Lin, Y.-L.; Yang, W.-C. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement. Altern. Med. 2013, 13, 45. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.O.; Kim, B.Y.; Lee, M.H.; Kim, Y.R.; Chung, H.Y.; Park, J.H.; Moon, J.O. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2003, 55, 1695–1700. [Google Scholar] [CrossRef]
- Soonthornsit, N.; Pitaksutheepong, C.; Hemstapat, W.; Utaisincharoen, P.; Pitaksuteepong, T. In Vitro Anti-Inflammatory Activity of Morus alba L. Stem Extract in LPS-Stimulated RAW 264.7 Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 3928956. [Google Scholar] [CrossRef] [Green Version]
- Breuer, C.; Wolf, G.; Andrabi, S.A.; Lorenz, P.; Horn, T.F. Blood-brain barrier permeability to the neuroprotectant oxyresveratrol. Neurosci. Lett. 2006, 393, 113–118. [Google Scholar] [CrossRef]
- Wang, C.P.; Zhang, L.Z.; Li, G.C.; Shi, Y.W.; Li, J.L.; Zhang, X.C.; Wang, Z.W.; Ding, F.; Liang, X.M. Mulberroside A protects against ischemic impairment in primary culture of rat cortical neurons after oxygen-glucose deprivation followed by reperfusion. J. Neurosci. Res. 2014, 92, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Hasriadi; Wong-on, M.; Lapphanichayakool, P.; Limpeanchob, N. Neuroprotective Effect of Artocarpus Lakoocha Extract and Oxyresveratrol against Hydrogen Peroxide-Induced Toxicity in Sh-Sy5y Cells. Int.J. Pharm. Sci. 2017, 9, 229–233. [Google Scholar]
- Pullen, N.G. Thomas The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997, 410, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Leopold, S.J.; Cranendonk, D.R.; van der Poll, T. Host innate immune responses to sepsis. Virulence 2014, 5, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Lappin, E.; Ferguson, A.J. Gram-positive toxic shock syndromes. Lancet. Infect. Dis. 2009, 9, 281–290. [Google Scholar] [CrossRef]
- Fink, M.P.; Warren, H.S. Strategies to improve drug development for sepsis. Nat. Rev. Drug Discov. 2014, 13, 741–758. [Google Scholar] [CrossRef]
- Gutsmann, T.; Razquin-Olazaran, I.; Kowalski, I.; Kaconis, Y.; Howe, J.; Bartels, R.; Hornef, M.; Schurholz, T.; Rossle, M.; Sanchez-Gomez, S.; et al. New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob. Agents Chemother. 2010, 54, 3817–3824. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Guo, Z.; He, W.; Yang, Y.; Li, Y.; Zheng, A.; Li, P.; Zhang, Y.; Ma, J.; Wen, M.; et al. Ephedrine hydrochloride protects mice from LPS challenge by promoting IL-10 secretion and inhibiting proinflammatory cytokines. Int. Immunopharmacol. 2012, 13, 46–53. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Li, Y.; Xu, L.; Wang, Y.; Guo, Z.; Song, H.; Yang, M.; Luo, B.; Zheng, A.; et al. Ephedrine hydrochloride inhibits PGN-induced inflammatory responses by promoting IL-10 production and decreasing proinflammatory cytokine secretion via the PI3K/Akt/GSK3beta pathway. Cell Mol. Immunol. 2013, 10, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Na Takuathung, M.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement. Altern. Med. 2018, 18, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potikanond, S.; Sookkhee, S.; Na Takuathung, M.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia parviflora Extract Exhibits Anti-cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suradej, B.; Sookkhee, S.; Panyakaew, J.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Kaempferia parviflora Extract Inhibits STAT3 Activation and Interleukin-6 Production in HeLa Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phaosri, M.; Jantrapirom, S.; Takuathung, M.N.; Soonthornchareonnon, N.; Sireeratawong, S.; Buacheen, P.; Pitchakarn, P.; Nimlamool, W.; Potikanond, S. Salacia chinensis L. Stem Extract Exerts Antifibrotic Effects on Human Hepatic Stellate Cells Through the Inhibition of the TGF-beta1-Induced SMAD2/3 Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 6314. [Google Scholar] [CrossRef] [Green Version]
- Namsen, R.; Rojanasthien, N.; Sireeratawong, S.; Rojsanga, P.; Nimlamool, W.; Potikanond, S. Thunbergia laurifolia Exhibits Antifibrotic Effects in Human Hepatic Stellate Cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 3508569. [Google Scholar] [CrossRef] [Green Version]
- Maneechai, S.; De-Eknamkul, W.; Umehara, K.; Noguchi, H.; Likhitwitayawuid, K. Flavonoid and stilbenoid production in callus cultures of Artocarpus lakoocha. Phytochemistry 2012, 81, 42–49. [Google Scholar] [CrossRef]
- Pandey, A.; Bhatnagar, S.P. Preliminary Phytochemical screening and antimicrobial studies on Artocarpus lakoocha Roxb. Anc. Sci. Life 2009, 28, 21–24. [Google Scholar]
- Tengamnuay, P.; Pengrungruangwong, K.; Pheansri, I.; Likhitwitayawuid, K. Artocarpus lakoocha heartwood extract as a novel cosmetic ingredient: Evaluation of the in vitro anti-tyrosinase and in vivo skin whitening activities. Int. J. Cosmet. Sci. 2006, 28, 269–276. [Google Scholar] [CrossRef]
- Leon, L.R.; White, A.A.; Kluger, M.J. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 1998, 275, R269–R277. [Google Scholar] [CrossRef]
- Geppert, A.; Steiner, A.; Zorn, G.; Delle-Karth, G.; Koreny, M.; Haumer, M.; Siostrzonek, P.; Huber, K.; Heinz, G. Multiple organ failure in patients with cardiogenic shock is associated with high plasma levels of interleukin-6. Crit. Care Med. 2002, 30, 1987–1994. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Bolanos, N.; Laoutaris, G.; Papadakis, V.; Koussoulas, V.; Perrea, D.; Karayannacos, P.E.; Giamarellou, H. Immunomodulatory intervention in sepsis by multidrug-resistant Pseudomonas aeruginosa with thalidomide: An experimental study. BMC Infect. Dis. 2005, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu. Rev. Immunol. 2015, 33, 257–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.W.; Heldwein, K.A.; Means, T.K.; Saukkonen, J.J.; Fenton, M.J. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann. Rheum. Dis. 2001, 60, 6–12. [Google Scholar]
- Beinke, S.; Ley, S.C. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 2004, 382, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Wadleigh, D.J.; Reddy, S.T.; Kopp, E.; Ghosh, S.; Herschman, H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem. 2000, 275, 6259–6266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [PubMed]
- Kim, J.W.; Kim, C. Inhibition of LPS-induced NO production by taurine chloramine in macrophages is mediated though Ras-ERK-NF-kappaB. Biochem. Pharmacol. 2005, 70, 1352–1360. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hankittichai, P.; Buacheen, P.; Pitchakarn, P.; Na Takuathung, M.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Artocarpus lakoocha Extract Inhibits LPS-Induced Inflammatory Response in RAW 264.7 Macrophage Cells. Int. J. Mol. Sci. 2020, 21, 1355. https://doi.org/10.3390/ijms21041355
Hankittichai P, Buacheen P, Pitchakarn P, Na Takuathung M, Wikan N, Smith DR, Potikanond S, Nimlamool W. Artocarpus lakoocha Extract Inhibits LPS-Induced Inflammatory Response in RAW 264.7 Macrophage Cells. International Journal of Molecular Sciences. 2020; 21(4):1355. https://doi.org/10.3390/ijms21041355
Chicago/Turabian StyleHankittichai, Phateep, Pensiri Buacheen, Pornsiri Pitchakarn, Mingkwan Na Takuathung, Nitwara Wikan, Duncan R. Smith, Saranyapin Potikanond, and Wutigri Nimlamool. 2020. "Artocarpus lakoocha Extract Inhibits LPS-Induced Inflammatory Response in RAW 264.7 Macrophage Cells" International Journal of Molecular Sciences 21, no. 4: 1355. https://doi.org/10.3390/ijms21041355
APA StyleHankittichai, P., Buacheen, P., Pitchakarn, P., Na Takuathung, M., Wikan, N., Smith, D. R., Potikanond, S., & Nimlamool, W. (2020). Artocarpus lakoocha Extract Inhibits LPS-Induced Inflammatory Response in RAW 264.7 Macrophage Cells. International Journal of Molecular Sciences, 21(4), 1355. https://doi.org/10.3390/ijms21041355