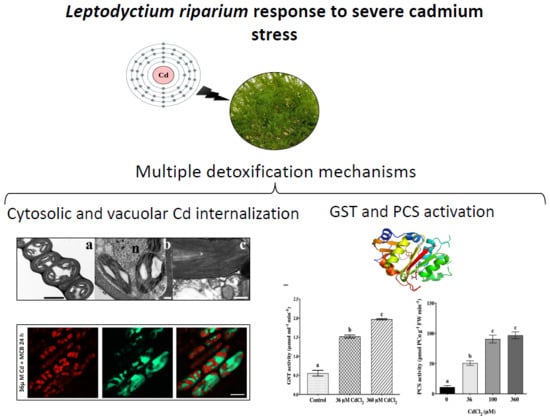
The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins
Abstract
:1. Introduction
2. Results
2.1. ROS Production and Antioxidant Response to Cd
2.2. L. riparium Possesses a Functional PCS that Produces Cd-Induced PCn in Vitro and in Vivo
2.3. Confocal Imaging of MCB Staining and Chlorophyll Autofluorescence
2.4. Cd Treatments Caused Only Slight Cytohistological Damage to Gametophytes
2.5. Cd Treatment Lowers Photosynthetic Activity in L. riparium Gametophytes
2.6. Ultrastructural Observations Evidenced Slight Ultrastructure Alteration in Cd-Exposed Gametophytes
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Detection of ROS Production and SOD, CAT, and GST Activities
4.3. In Vitro Activity Assay of PCS
4.4. γ–EC, GSH, and PCn Extraction, Characterization, and Quantification
4.5. Confocal Laser Imaging of MCB Internalization
4.6. Evans Blue Staining and Microscopy
4.7. Photochemical Efficiency
4.8. Ultrastructural Observations
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCn | Phytochelatins |
| PCS | Phytochelatin synthase |
| GSH | Glutathione |
| GS–bimane | Glutathione–bimane |
| γ–EC | γ–glutamylcysteine |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GST | Glutathione-S–transferase |
| MCB | Monochlorobimane |
References
- Sanità di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Vassilev, A.; Yordanov, I.; Vangronsveld, J. Cadmium phytoextraction from contaminated soils. In Cadmium Toxicity and Tolerance in Plants; Khan, N.A., Samiullah, Y., Eds.; Narosa Publishers: New Delhi, India, 2006; pp. 35–61. [Google Scholar]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendry, G.A.F.; Baker, A.J.M.; Ewart, C.F. Cadmium tolerance and toxicity, oxygen radical processes and molecular damage in cadmium-tolerant and cadmium-sensitive clones of Holcus lanatus L. Acta Bot. Neerl. 1992, 41, 271–281. [Google Scholar] [CrossRef]
- Somashekaraiah, B.V.; Padmaja, K.; Prasad, A.R.K. Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): Involvement of lipid peroxides in chlorphyll degradation. Physiol. Plantarum. 1992, 85, 85–89. [Google Scholar] [CrossRef]
- Salin, M.L. Toxic oxygen species and protective systems of the chloroplast. Physiol. Plantarum. 1988, 72, 681–689. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Adamis, P.D.B.; Gomes, D.S.; Pinto, M.L.C.C.; Panek, A.D.; Eleutherio, E.C.A. The role of glutathione transferases in cadmium stress. Toxicol. Lett. 2004, 154, 81–88. [Google Scholar] [CrossRef]
- Moons, A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal- and hypoxic stress-induced and differentially salt stress-responsive in rice roots 1. FEBS Lett. 2003, 553, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L.) O. Kuntze. Environ. Toxicol. 2007, 22, 368–374. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, X.; Gao, K.; Ge, Y.; Cheng, W. Non-protein thiols and glutathione S-transferase alleviate Cd stress and reduce root-to-shoot translocation of Cd in rice. J. Plant Nutr. Soil Sci. 2013, 176, 626–633. [Google Scholar] [CrossRef]
- Grzam, A.; Tennstedt, P.; Clemens, S.; Hell, R.; Meyer, A.J. Vacuolar sequestration of glutathione S-conjugates outcompetes a possible degradation of the glutathione moiety by phytochelatin synthase. FEBS Lett. 2006, 580, 6384–6390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rea, P.A.; Li, Z.-S.; Lu, Y.-P.; Drozdowicz, Y.M.; Martinoia, E. From vacuolar GS-X pumps to multispecific ABC transporters. Annu. Rev. Plant Physiol. 1998, 49, 727–760. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.J.; May, M.J.; Fricker, M. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J. 2001, 27, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasternak, M.; Lim, B.; Wirtz, M.; Hell, R.; Cobbett, C.S.; Meyer, A.J. Restricting glutathione biosynthesis to the cytosol is sufficient for normal plant development. Plant J. 2008, 53, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins: The principal heavy-metal complexing peptides of higher plants. Science 1985, 230, 674–676. [Google Scholar] [CrossRef]
- Clemens, S.; Peršoh, D. Multi-tasking phytochelatin synthases. Plant Sci. 2009, 177, 266–271. [Google Scholar] [CrossRef]
- Loeffler, S.; Hochberger, A.; Grill, E.; Winnacker, E.-L.; Zenk, M.H. Termination of the phytochelatin synthase reaction through sequestration of heavy metals by the reaction product. FEBS Lett. 1989, 258, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.; Lendzian, K.; Oven, M.; Christmann, A.; Grill, E. Phytochelatin synthase catalyzes key step in turnover of glutathione conjugates. Phytochemistry 2003, 62, 423–431. [Google Scholar] [CrossRef]
- Blum, R.; Beck, A.; Korte, A.; Stengel, A.; Letzel, T.; Lendzian, K.; Grill, E. Function of phytochelatin synthase in catabolism of glutathione-conjugates. Plant J. 2007, 49, 740–749. [Google Scholar] [CrossRef]
- Blum, R.; Meyer, K.C.; Wünschmann, J.; Lendzian, K.J.; Grill, E. Cytosolic action of phytochelatin synthase. Plant Physiol. 2010, 153, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderpoorten, A.; Goffinet, B. Introduction to Bryophytes; Cambridge University Press: Cambridge, UK, 2009; ISBN 978-1-107-37736-3. [Google Scholar]
- Qiu, Y.-L.; Li, L.; Wang, B.; Chen, Z.; Knoop, V.; Groth-Malonek, M.; Dombrovska, O.; Lee, J.; Kent, L.; Rest, J.; et al. The deepest divergences in land plants inferred from phylogenomic evidence. Proc. Natl. Acad. Sci. USA 2006, 103, 15511–15516. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.-L. Phylogeny and evolution of charophytic algae and land plants. J. Syst. Evol. 2008, 46, 287–306. [Google Scholar]
- Petraglia, A.; De Benedictis, M.; Degola, F.; Pastore, G.; Calcagno, M.; Ruotolo, R.; Mengoni, A.; Sanità di Toppi, L. The capability to synthesize phytochelatins and the presence of constitutive and functional phytochelatin synthases are ancestral (plesiomorphic) characters for basal land plants. J. Exp. Bot. 2014, 65, 1153–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-responsive phytochelatin synthase is constitutively present in the ancient liverwort Lunularia cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmens, H.; Ilyin, I.; Mills, G.; Aboal, J.R.; Alber, R.; Blum, O.; Coşkun, M.; De Temmerman, L.; Fernández, J.Á.; Figueira, R.; et al. Country-specific correlations across Europe between modelled atmospheric cadmium and lead deposition and concentrations in mosses. Environ. Pollut. 2012, 166, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, A.; Sorbo, S.; Conte, B.; Cobianchi, R.C.; Trinchella, F.; Capasso, C.; Carginale, V. Toxicity, accumulation, and removal of heavy metals by three aquatic macrophytes. Int. J. Phytoremediat. 2012, 14, 374–387. [Google Scholar] [CrossRef]
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Karam, E.A.; Lentini, M.; et al. In-field and in-vitro study of the moss Leptodictyum riparium as bioindicator of toxic metal pollution in the aquatic environment: Ultrastructural damage, oxidative stress and HSP70 induction. PLoS ONE 2018, 13, e0195717. [Google Scholar] [CrossRef]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxycol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Pisani, T.; Paoli, L.; Munzi, S.; Loppi, S. Bioacumulation and ultrastructural effects of Cd, Cu, Pb and Zn in the moss Scorpiurum circinatum (Brid.) Fleisch. & Loeske. Environ. Pollut. 2012, 166, 208–211. [Google Scholar] [PubMed]
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of heavy Metals on ultrastructure and HSP70S induction in the aquatic moss Leptodictyum riparium Hedw. Int. J. Phytoremediat. 2012, 14, 443–455. [Google Scholar] [CrossRef]
- Rea, P.A. Phytochelatin synthase: Of a protease a peptide polymerase made. Physiol. Plantarum. 2012, 145, 154–164. [Google Scholar] [CrossRef]
- Bleuel, C.; Wesenberg, D.; Meyer, A.J. Degradation of glutathione S-conjugates in Physcomitrella patens is initiated by cleavage of glycine. Plant Cell Physiol. 2011, 52, 1153–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruns, I.; Friese, K.; Markert, B.; Krauss, G.-J. Heavy metal inducible compounds from Fontinalis antipyretica reacting with Ellman’s reagent are not phytochelatins. Sci. Total Environ. 1999, 241, 215–216. [Google Scholar] [CrossRef]
- Wójcik, M.; Tukiendorf, A. Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biol. Plant 2011, 55, 125–132. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Han, X.-M.; Ren, L.-L.; Yang, H.-L.; Zeng, Q.-Y. Functional divergence of the glutathione S-transferase supergene family in Physcomitrella patens reveals complex patterns of large gene family evolution in land plants. Plant Physiol. 2013, 161, 773–786. [Google Scholar] [CrossRef] [Green Version]
- Huber, C.; Bartha, B.; Harpaintner, R.; Schröder, P. Metabolism of acetaminophen (paracetamol) in plants—two independent pathways result in the formation of a glutathione and a glucose conjugate. Environ. Sci. Pollut. Res. 2009, 16, 206. [Google Scholar] [CrossRef] [Green Version]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [Green Version]
- Grzam, A.; Martin, M.N.; Hell, R.; Meyer, A.J. γ-glutamyl transpeptidase GGT4 initiates vacuolar degradation of glutathione S-conjugates in Arabidopsis. FEBS Lett. 2007, 581, 3131–3138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohkama-Ohtsu, N.; Zhao, P.; Xiang, C.; Oliver, D.J. Glutathione conjugates in the vacuole are degraded by γ-glutamyl transpeptidase GGT3 in Arabidopsis. Plant J. 2007, 49, 878–888. [Google Scholar] [CrossRef] [PubMed]
- French, C.E.; Bell, J.M.L.; Ward, F.B. Diversity and distribution of hemerythrin-like proteins in prokaryotes. FEMS Microbiol. Lett. 2008, 279, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBel, C.P.; Ali, S.F.; McKee, M.; Bondy, S.C. Organometal-induced increases in oxygen reactive species: The potential of 2′, 7′-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol. Appl. Pharm. 1990, 104, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Glutathione S-transferases (rat and human). Methods in Enzymology. In Detoxication and Drug Metabolism: Conjugation and Related Systems; Academic Press: Cambridge, MA, USA, 1981; Volume 77, pp. 218–231. [Google Scholar]
- Wojas, S.; Clemens, S.; Hennig, J.; Sklodowska, A.; Kopera, E.; Schat, H.; Bal, W.; Antosiewicz, D.M. Overexpression of phytochelatin synthase in tobacco: Distinctive effects of AtPCS1 and CePCS genes on plant response to cadmium. J. Exp. Bot. 2008, 59, 2205–2219. [Google Scholar] [CrossRef] [Green Version]
- Bellini, E.; Borsò, M.; Betti, C.; Bruno, L.; Andreucci, A.; Ruffini Castiglione, M.; Saba, A.; Sanità di Toppi, L. Characterization and quantification of thiol-peptides in Arabidopsis thaliana using combined dilution and high sensitivity HPLC-ESI-MS-MS. Phytochemistry 2019, 164, 215–222. [Google Scholar] [CrossRef]
- Ponce de León, I.; Oliver, J.P.; Castro, A.; Gaggero, C.; Bentancor, M.; Vidal, S. Erwinia carotovora elicitors and Botrytis cinerea activate defense responses in Physcomitrella patens. BMC Plant Biol. 2007, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Basile, A.; Cogoni, A.E.; Bassi, P.; Fabrizi, E.; Sorbo, S.; Giordano, S.; Castaldo Cobianchi, R. Accumulation of Pb and Zn in gametophytes and sporophytes of the moss Funaria hygrometrica (Funariales). Ann. Bot. 2001, 87, 537–543. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S.; et al. The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. https://doi.org/10.3390/ijms21051583
Bellini E, Maresca V, Betti C, Castiglione MR, Fontanini D, Capocchi A, Sorce C, Borsò M, Bruno L, Sorbo S, et al. The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. International Journal of Molecular Sciences. 2020; 21(5):1583. https://doi.org/10.3390/ijms21051583
Chicago/Turabian StyleBellini, Erika, Viviana Maresca, Camilla Betti, Monica Ruffini Castiglione, Debora Fontanini, Antonella Capocchi, Carlo Sorce, Marco Borsò, Laura Bruno, Sergio Sorbo, and et al. 2020. "The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins" International Journal of Molecular Sciences 21, no. 5: 1583. https://doi.org/10.3390/ijms21051583
APA StyleBellini, E., Maresca, V., Betti, C., Castiglione, M. R., Fontanini, D., Capocchi, A., Sorce, C., Borsò, M., Bruno, L., Sorbo, S., Basile, A., & Sanità di Toppi, L. (2020). The Moss Leptodictyum riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. International Journal of Molecular Sciences, 21(5), 1583. https://doi.org/10.3390/ijms21051583