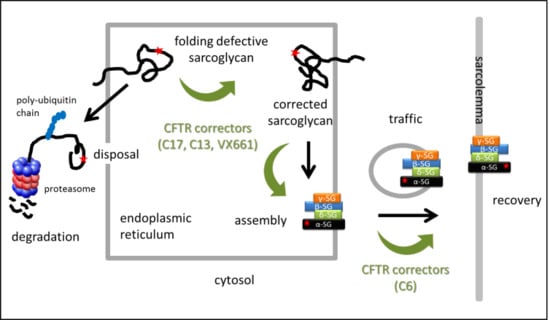
Combined Use of CFTR Correctors in LGMD2D Myotubes Improves Sarcoglycan Complex Recovery
Abstract
:1. Introduction
2. Results
2.1. Rescue of α-SG by CFTR Correctors in LGMD2D Myotubes
2.2. Additive Effect of C13 in Combination with C17
2.3. Additive/Synergistic Effects of C6 in Combination with C17
2.4. CFTR Correctors Are Effective Only on V247M-α-SG Mutant
2.5. Assembly of the Rescued α-SG into the Sarcoglycan Complex
2.6. The Rescued Protein Is Stable at the Plasma Membrane
3. Discussion
4. Material and Methods
4.1. Site Directed Mutagenesis
4.2. Chemicals, Cell Culture and Treatments
4.3. Biotinylation of Surface Proteins
4.4. Co-Immunoprecipitation
4.5. Western Blot Analysis
4.6. Antibodies
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Straub, V.; Murphy, A.; Udd, B. Group lws: 229th ENMC international workshop: Limb girdle muscular dystrophies—Nomenclature and reformed classification Naarden, the Netherlands, 17–19 March 2017. Neuromuscul. Disord. 2018, 28, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushby, K.; Norwood, F.; Straub, V. The limb-girdle muscular dystrophies—Diagnostic strategies. Bba Mol. Basis Dis. 2007, 1772, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Romero, N.B.; Tome, F.M.S.; Leturcq, F.; Elkerch, F.; Azibi, K.; Bachner, L.; Anderson, R.D.; Roberds, S.L.; Campbell, K.P.; Fardeau, M.; et al. Genetic-heterogeneity of severe childhood autosomal recessive muscular-dystrophy with adhalin (50 Kda dystrophy-associated glycoprotein) deficiency. Comptes Rendus Acad. Sci. Ser. III Sci. Vie 1994, 317, 70–76. [Google Scholar]
- Allamand, V.; Leturcq, F.; Piccolo, F.; Jeanpierre, M.; Azibi, K.; Roberds, S.L.; Lim, L.E.; Campbell, K.P.; Beckmann, J.S.; Kaplan, J.C. Adhalin gene polymorphism. Hum. Mol. Genet. 1994, 3, 2269. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Savarese, M. Genetic basis of limb-girdle muscular dystrophies: the 2014 update. Acta Myol. 2014, 33, 1–12. [Google Scholar]
- Sandona, D.; Betto, R. Sarcoglycanopathies: Molecular pathogenesis and therapeutic prospects. Expert Rev. Mol. Med. 2009, 11, e28. [Google Scholar] [CrossRef]
- Tarakci, H.; Berger, J. The sarcoglycan complex in skeletal muscle. Front. Biosci. 2016, 21, 744–756. [Google Scholar]
- Kirschner, J.; Lochmuller, H. Sarcoglycanopathies. Handb. Clin. Neurol. 2011, 101, 41–46. [Google Scholar]
- Carotti, M.; Fecchio, C.; Sandona, D. Emerging therapeutic strategies for sarcoglycanopathy. Expert Opin. Orphan Drugs 2017, 5, 381–396. [Google Scholar] [CrossRef]
- Ginjaar, H.B.; van der Kooi, A.J.; Ceelie, H.; Kneppers, A.L.; van Meegen, M.; Barth, P.G.; Busch, H.F.; Wokke, J.H.; Anderson, L.V.; Bonnemann, C.G.; et al. Sarcoglycanopathies in Dutch patients with autosomal recessive limb girdle muscular dystrophy. J. Neurol. 2000, 247, 524–529. [Google Scholar] [CrossRef]
- Vainzof, M.; Passos-Bueno, M.R.; Pavanello, R.C.; Marie, S.K.; Oliveira, A.S.; Zatz, M. Sarcoglycanopathies are responsible for 68% of severe autosomal recessive limb-girdle muscular dystrophy in the Brazilian population. J. Neurol. Sci. 1999, 164, 44–49. [Google Scholar] [CrossRef]
- Duggan, D.J.; Gorospe, J.R.; Fanin, M.; Hoffman, E.P.; Angelini, C.; Pegoraro, E.; Noguchi, S.; Ozawa, E.; Pendlebury, W.; Waclawik, A.J.; et al. Mutations in the sarcoglycan genes in patients with myopathy. N. Engl. J. Med. 1997, 336, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Hou, Y.; Yu, M.; Liu, Y.; Fan, Y.; Zhang, W.; Wang, Z.; Xiong, H.; Yuan, Y. Clinical and genetic spectrum of sarcoglycanopathies in a large cohort of Chinese patients. Orphanet J. Rare Dis. 2019, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastaldello, S.; D’Angelo, S.; Franzoso, S.; Fanin, M.; Angelini, C.; Betto, R.; Sandona, D. Inhibition of proteasome activity promotes the correct localization of disease-causing alpha-sarcoglycan mutants in HEK-293 cells constitutively expressing beta-, gamma-, and delta-sarcoglycan. Am. J. Pathol. 2008, 173, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, M.; Gicquel, E.; Barrault, L.; Soheili, T.; Malissen, M.; Malissen, B.; Vincent-Lacaze, N.; Perez, N.; Udd, B.; Danos, O.; et al. Mannosidase I inhibition rescues the human alpha-sarcoglycan R77C recurrent mutation. Hum. Mol. Genet. 2008, 17, 1214–1221. [Google Scholar] [CrossRef]
- Soheili, T.; Gicquel, E.; Poupiot, J.; N’Guyen, L.; Le Roy, F.; Bartoli, M.; Richard, I. Rescue of sarcoglycan mutations by inhibition of endoplasmic reticulum quality control is associated with minimal structural modifications. Hum. Mutat. 2012, 33, 429–439. [Google Scholar] [CrossRef]
- Bianchini, E.; Fanin, M.; Mamchaoui, K.; Betto, R.; Sandona, D. Unveiling the degradative route of the V247M alpha-sarcoglycan mutant responsible for LGMD-2D. Hum. Mol. Genet. 2014, 23, 3746–3758. [Google Scholar] [CrossRef]
- Gelman, M.S.; Kannegaard, E.S.; Kopito, R.R. A principal role for the proteasome in endoplasmic reticulum-associated degradation of misfolded intracellular cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 2002, 277, 11709–11714. [Google Scholar] [CrossRef] [Green Version]
- Carotti, M.; Marsolier, J.; Soardi, M.; Bianchini, E.; Gomiero, C.; Fecchio, C.; Henriques, S.F.; Betto, R.; Sacchetto, R.; Richard, I.; et al. Repairing folding-defective alpha-sarcoglycan mutants by CFTR correctors, a potential therapy for limb-girdle muscular dystrophy 2D. Hum. Mol. Genet. 2018, 27, 969–984. [Google Scholar] [CrossRef] [Green Version]
- Rogan, M.P.; Stoltz, D.A.; Hornick, D.B. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest 2011, 139, 1480–1490. [Google Scholar] [CrossRef]
- Birault, V.; Solari, R.; Hanrahan, J.; Thomas, D.Y. Correctors of the basic trafficking defect of the mutant F508del-CFTR that causes cystic fibrosis. Curr. Opin. Chem. Biol. 2013, 17, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sabirzhanova, I.; Pacheco, M.L.; Rapino, D.; Grover, R.; Handa, J.T.; Guggino, W.B.; Cebotaru, L. Rescuing trafficking mutants of the ATP-binding cassette protein, ABCA4, with small molecule correctors as a treatment for stargardt eye disease. J. Biol. Chem. 2015, 290, 19743–19755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, H.M.; Lam, H.; Chen, P.C.; Zhang, D.L.; Mottillo, C.; Mirza, M.; Qasim, K.; Shrier, A.; Shyng, S.L.; Hanrahan, J.W.; et al. Compounds that correct F508del-CFTR trafficking can also correct other protein trafficking diseases: An In Vitro study using cell lines. Orphanet J. Rare Dis. 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Woerd, W.L.; Wichers, C.G.K.; Vestergaard, A.L.; Andersen, J.P.; Paulusma, C.C.; Houwen, R.H.J.; van de Graaf, S.F.J. Rescue of defective ATP8B1 trafficking by CFTR correctors as a therapeutic strategy for familial intrahepatic cholestasis. J. Hepatol. 2016, 64, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-Moran, O.; Galietta, L.J.; Verkman, A.S. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef] [PubMed]
- Robert, R.; Carlile, G.W.; Pavel, C.; Liu, N.; Anjos, S.M.; Liao, J.; Luo, Y.; Zhang, D.L.; Thomas, D.Y.; Hanrahan, J.W. Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Mol. Pharmacol. 2008, 73, 478–489. [Google Scholar] [CrossRef] [Green Version]
- Connett, G.J. Lumacaftor-ivacaftor in the treatment of cystic fibrosis: Design, development and place in therapy. Drug Des. Dev. Ther. 2019, 13, 2405–2412. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; Mckee, C.; Lu, Y.M.; et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Thorley, M.; Duguez, S.; Mazza, E.M.C.; Valsoni, S.; Bigot, A.; Mamchaoui, K.; Harmon, B.; Voit, T.; Mouly, V.; Duddy, W. Skeletal muscle characteristics are preserved in hTERT/cdk4 human myogenic cell lines. Skelet. Muscle 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.L.; Yu, G.J.; Yang, B.; Robins, L.I.; Verkman, A.S.; Kurth, M.J. 4′-methyl-4,5′-bithiazole-based correctors of defective Delta F508-CFTR cellular processing. Bioorg. Med. Chem. Lett. 2008, 18, 2610–2614. [Google Scholar] [CrossRef] [Green Version]
- Noack, J.; Pisoni, G.B.; Molinari, M. Proteostasis: Bad news and good news from the endoplasmic reticulum. Swiss Med. Wkly. 2014, 144, w14001. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Verkman, A.S. Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb. Perspect. Med. 2013, 3, a009761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Chen, Z.; Schottenfeld, J.; Stahl, R.C.; Kunkel, L.M.; Chan, Y.M. Specific assembly pathway of sarcoglycans is dependent on beta- and delta-sarcoglycan. Muscle Nerve 2004, 29, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Draviam, R.A.; Shand, S.H.; Watkins, S.C. The beta-delta-core of sarcoglycan is essential for deposition at the plasma membrane. Muscle Nerve 2006, 34, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Allikian, M.J.; McNally, E.M. Processing and assembly of the dystrophin glycoprotein complex. Traffic 2007, 8, 177–183. [Google Scholar] [CrossRef]
- Sicari, D.; Igbaria, A.; Chevet, E. Control of protein homeostasis in the early secretory pathway: Current status and challenges. Cells 2019, 8, 1347. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.M.; Oster, M.E.; Hebert, D.N. Protein quality control in the endoplasmic reticulum. Protein J. 2019, 38, 317–329. [Google Scholar] [CrossRef]
- Balch, W.E.; Roth, D.M.; Hutt, D.M. Emergent properties of proteostasis in managing cystic fibrosis. Cold Spring Harb. Perspect. Biol. 2011, 3, a004499. [Google Scholar] [CrossRef] [Green Version]
- Hutt, D.M.; Powers, E.T.; Balch, W.E. The proteostasis boundary in misfolding diseases of membrane traffic. FEBS Lett. 2009, 583, 2639–2646. [Google Scholar] [CrossRef] [Green Version]
- Mijnders, M.; Kleizen, B.; Braakman, I. Correcting CFT folding defects by small-molecule correctors to cure cystic fibrosis. Curr. Opin. Pharmacol. 2017, 34, 83–90. [Google Scholar] [CrossRef]
- Noguchi, S.; Wakabayashi, E.; Imamura, M.; Yoshida, M.; Ozawa, E. Formation of sarcoglycan complex with differentiation in cultured myocytes. Eur. J. Biochem. 2000, 267, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okiyoneda, T.; Veit, G.; Dekkers, J.F.; Bagdany, M.; Soya, N.; Xu, H.J.; Roldan, A.; Verkman, A.S.; Kurth, M.; Simon, A.; et al. Mechanism-based corrector combination restores Delta F508-CFTR folding and function. Nat. Chem. Biol. 2013, 9, 444–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Pacheco, M.; Sabirzhanova, I.; Rapino, D.; Morales, M.M.; Guggino, W.B.; Cebotaru, L. Correctors rescue CFTR mutations in nucleotide-binding domain 1 (NBD1) by modulating proteostasis. Chembiochem 2016, 17, 493–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Pacheco, M.; Boinot, C.; Sabirzhanova, I.; Rapino, D.; Cebotaru, L. Combination of correctors rescues CFTR transmembrane-domain mutants by mitigating their interactions with proteostasis. Cell Physiol. Biochem. 2017, 41, 2194–2210. [Google Scholar] [CrossRef] [Green Version]
- Cant, N.; Pollock, N.; Ford, R.C. CFTR structure and cystic fibrosis. Int. J. Biochem. Cell Biol. 2014, 52, 15–25. [Google Scholar] [CrossRef]
- Dickens, N.J.; Beatson, S.; Ponting, C.P. Cadherin-like domains in alpha-dystroglycan, alpha/epsilon-sarcoglycan and yeast and bacterial proteins. Curr. Biol. 2002, 12, R197–R199. [Google Scholar] [CrossRef] [Green Version]
- Cebotaru, L.; Liu, Q.N.; Sabirzhanova, I.; Bergbower, E.; Yanda, M.; Guggino, W. The CFTR corrector, VX-809 (Lumacaftor) and Hsp27 rescues ABCA4 trafficking mutants: A potential treatment for Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, 218. [Google Scholar]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Sandona, D.; Gastaldello, S.; Martinello, T.; Betto, R. Characterization of the ATP-hydrolysing activity of alpha-sarcoglycan. Biochem. J. 2004, 381 Pt 1, 105–112. [Google Scholar] [CrossRef]
- Zhu, C.H.; Mouly, V.; Cooper, R.N.; Mamchaoui, K.; Bigot, A.; Shay, J.W.; Di Santo, J.P.; Butler-Browne, G.S.; Wright, W.E. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 2007, 6, 515–523. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carotti, M.; Scano, M.; Fancello, I.; Richard, I.; Risato, G.; Bensalah, M.; Soardi, M.; Sandonà, D. Combined Use of CFTR Correctors in LGMD2D Myotubes Improves Sarcoglycan Complex Recovery. Int. J. Mol. Sci. 2020, 21, 1813. https://doi.org/10.3390/ijms21051813
Carotti M, Scano M, Fancello I, Richard I, Risato G, Bensalah M, Soardi M, Sandonà D. Combined Use of CFTR Correctors in LGMD2D Myotubes Improves Sarcoglycan Complex Recovery. International Journal of Molecular Sciences. 2020; 21(5):1813. https://doi.org/10.3390/ijms21051813
Chicago/Turabian StyleCarotti, Marcello, Martina Scano, Irene Fancello, Isabelle Richard, Giovanni Risato, Mona Bensalah, Michela Soardi, and Dorianna Sandonà. 2020. "Combined Use of CFTR Correctors in LGMD2D Myotubes Improves Sarcoglycan Complex Recovery" International Journal of Molecular Sciences 21, no. 5: 1813. https://doi.org/10.3390/ijms21051813
APA StyleCarotti, M., Scano, M., Fancello, I., Richard, I., Risato, G., Bensalah, M., Soardi, M., & Sandonà, D. (2020). Combined Use of CFTR Correctors in LGMD2D Myotubes Improves Sarcoglycan Complex Recovery. International Journal of Molecular Sciences, 21(5), 1813. https://doi.org/10.3390/ijms21051813