Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings
Abstract
:1. Introduction
2. Results and Discussion
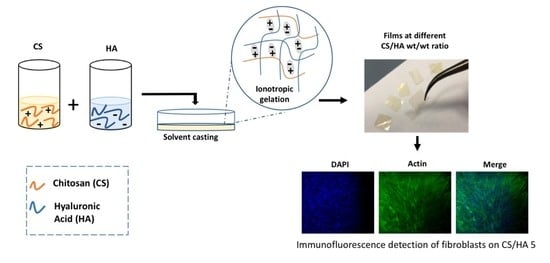
2.1. Physico-Chemical Characterization of CS/HA 2D-Matrices
2.2. Biological Characterization of CS/HA 2D-Matrices
3. Materials and Methods
3.1. Preparation of Chitosan–Hyaluronic Acid 2D-Matrices
3.2. SEM Observations
3.3. Film Transparency
3.4. Water-Uptake Capacity and Soluble Fraction
3.5. Water Vapor Transmission Rate Test
3.6. Static Contact Angle Measurement
3.7. Mechanical Characterization
3.8. Evaluation of Staphylococcus epidermidis Adhesion onto CS/HA Matrices
3.9. Cell Culture
3.9.1. Sample Preparation
3.9.2. Cell Viability
3.9.3. Immunofluorescence
3.10. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Oliveira, A.; Simões, S.; Ascenso, A.; Reis, C.P. Therapeutic Advances in Wound Healing. J. Dermatolog. Treat. 2020, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.X.; Mao, J.J.; Cheng, Y.; Liu, H.R.; Lv, L.; Ge, M.Z.; Li, S.H.; Huang, J.Y.; Chen, Z.; Li, H.Q.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef] [Green Version]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int. 2019, 35, 50–57. [Google Scholar]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Vuotto, C.; Donelli, G.; Lipsky, B.A. Biofilms and Wounds: An Identification Algorithm and Potential Treatment Options. Adv. Wound Care 2015, 4, 389–397. [Google Scholar] [CrossRef]
- Francolini, I.; Donelli, G.; Crisante, F.; Taresco, V.; Piozzi, A. Antimicrobial polymers for anti-biofilm medical devices: State-of-art and perspectives. Adv. Exp. Med. Biol. 2015, 831, 93–117. [Google Scholar]
- Taresco, V.; Crisante, F.; Francolini, I.; Martinelli, A.; D’Ilario, L.; Ricci-Vitiani, L.; Buccarelli, M.; Pietrelli, L.; Piozzi, A. Antimicrobial and antioxidant amphiphilic random copolymers to address medical device-centered infections. Acta Biomater. 2015, 22, 131–140. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef] [Green Version]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Biophys. Rev. 2019, 11, 807–815. [Google Scholar] [CrossRef]
- Amato, A.; Migneco, L.M.; Martinelli, A.; Pietrelli, L.; Piozzi, A.; Francolini, I. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr. Polym. 2018, 179, 273–281. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Zhao, L. Antibacterial activity and mechanism of chitosan with ultra high molecular weight. Carbohydr. Polym. 2016, 148, 200–205. [Google Scholar] [CrossRef]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef]
- Pietrelli, L.; Francolini, I.; Piozzi, A. Dyes Adsorption from Aqueous Solutions by Chitosan. Sep. Sci. Technol. 2015, 50, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Francolini, I.; Perugini, E.; Silvestro, I.; Lopreiato, M.; Scotto d’Abusco, A.; Valentini, F.; Placidi, E.; Arciprete, F.; Martinelli, A.; Piozzi, A. Graphene Oxide Oxygen Content Affects Physical and Biological Properties of Scaffolds Based on Chitosan/Graphene Oxide Conjugates. Materials 2019, 12, 1142. [Google Scholar] [CrossRef] [Green Version]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, D. Biopolymer Microparticles Prepared by Microfluidics for Biomedical Applications. Small 2020, 16, 1903736. [Google Scholar] [CrossRef]
- De Masi, A.; Tonazzini, I.; Masciullo, C.; Mezzena, R.; Chiellini, F.; Puppi, D.; Cecchini, M. Chitosan films for regenerative medicine: Fabrication methods and mechanical characterization of nanostructured chitosan films. Biophys. Rev. 2019, 11, 807–815. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- CuzzucoliCrucitti, V.; Migneco, L.M.; Piozzi, A.; Taresco, V.; Garnett, M.; Argent, R.H.; Francolini, I. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur. J. Pharm. Biopharm. 2018, 125, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Sarasam, A.; Madihally, S.V. Characterization of chitosan-polycaprolactone blends for tissue engineering applications. Biomaterials 2005, 26, 5500–5508. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch-chitosan blend biodegradable film. LWT-Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Iacob, A.T.; Drăgan, M.; Ghețu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 86, 103–108. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Aly, A.A.; Abou-Okeil, A. A non-woven fabric wound dressing containing layer-by-layer deposited hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2018, 114, 929–934. [Google Scholar] [CrossRef]
- Xu, H.T.; Ma, L.; Shi, H.F.; Gao, C.Y.; Han, C.M. Chitosan-hyaluronic acid hybrid film as a novel wound dressing: In vitro and in vivo studies. Polym. Adv. Technol. 2007, 18, 869–875. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; MohyEldin, M.S.; Šoltés, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 227–235. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Fohlerova, Z.; Pavliňák, D.; Sayed, O.N.; Jancar, J. Wound dressing based on chitosan/hyaluronan/nonwoven fabrics: Preparation, characterization and medical applications. Int. J. Biol. Macromol. 2016, 89, 725–736. [Google Scholar] [CrossRef]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Neuman, M.G.; Nanau, R.M.; Oruna-Sanchez, L.; Coto, G. Hyaluronic Acid and Wound Healing. J. Pharm. Pharm. Sci. 2015, 18, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Romanò, C.L.; De Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic acid and its composites as a local antimicrobial/antiadhesive barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef]
- Glibbery, A.; Mani, R. pH in leg ulcers. Int. J. Microcirc. Clin. Exp. 1992, 11, S109. [Google Scholar]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Lamke, L.O.; Nilsson, G.E.; Reithner, H.L. The evaporative water loss from burns and the water permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Queen, D.; Gaylor, J.D.S.; Evans, J.H.; Courtney, J.M.; Reid, W.H. The preclinical evaluation of the water-vapor transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y.; et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef] [Green Version]
- Hollinworth, H. Nurse’s assessment and management of pain at wound dressing changes. J. Wound Care 1995, 4, 77–83. [Google Scholar] [CrossRef]
- Francolini, I.; Silvestro, I.; Di Lisio, V.; Martinelli, A.; Piozzi, A. Synthesis, Characterization, and Bacterial Fouling-Resistance Properties of Polyethylene Glycol-Grafted Polyurethane Elastomers. Int. J. Mol. Sci. 2019, 20, 1001. [Google Scholar] [CrossRef] [Green Version]
- Francolini, I.; Donelli, G.; Vuotto, C.; Baroncini, F.A.; Stoodley, P.; Taresco, V.; Martinelli, A.; D’Ilario, L.; Piozzi, A. Antifouling polyurethanes to fight device-related staphylococcal infections: Synthesis, characterization, and antibiofilm efficacy. Pathog. Dis. 2014, 70, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Junter, G.A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.A.; Wu, H.G. Characterization of Chitosan-Hyaluronic Acid Blended Membranes and Their Effects on the Growth of Keratocytes. Polym. Polym. Compos. 2011, 19, 573–580. [Google Scholar] [CrossRef]
- Tzoneva, R.; Faucheux, N.; Groth, T. Wettability of substrata controls cell-substrate and cell-cell adhesions. Biochim. Biophys. Acta 2007, 1770, 1538–1547. [Google Scholar] [CrossRef]
- Faucheux, N.; Schweiss, R.; Lutzow, K.; Werner, C.; Groth, T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials 2004, 25, 2721–2730. [Google Scholar] [CrossRef]
- Lopreiato, M.; Cocchiola, R.; Falcucci, S.; Leopizzi, M.; Cardone, M.; Di Maio, V.; Brocco, U.; D’Orazi, V.; Calvieri, S.; Scandurra, R.; et al. The Glucosamine-derivative NAPA Suppresses MAPK Activation and Restores Collagen Deposition in Human Diploid Fibroblasts Challenged with Environmental Levels of UVB. Photochem. Photobiol. 2020, 96, 74–82. [Google Scholar] [CrossRef]









| Sample | HA Content (%) | Contact Angle (ϑ°) | Mechanical Properties | WVTR (g/day m2) | Staph. ep. CFUs/cm2 | ||
|---|---|---|---|---|---|---|---|
| E (GPa) | TS (MPa) | EB (%) | |||||
| CS | 0 | 98 ± 3 | 0.2 ± 0.04 | 5.0 ± 1 | 5 ± 1 | 576 | 9 × 107 |
| CS/HA1 | 1 | 96 ± 2 | 0.2 ± 0.03 | 4.3 ± 0.5 | 6 ± 1 | 595 | 4 × 107 |
| CS/HA5 | 5 | 93 ± 1 | 0.5 ± 0.1 | 40 ± 8 | 7 ± 1 | 672 | ND |
| CS/HA10 | 10 | 89 ± 2 | 2.0 ± 0.4 | 60 ± 12 | 7 ± 1 | 624 | 4.8 × 104 |
| CS/HA15 | 15 | 84 ± 1 | 1.5 ± 0.3 | 40 ± 7 | 8 ± 2 | 500 | 2.5 × 104 |
| CS/HA25 | 25 | 82 ± 2 | 2.0 ± 0.2 | 60 ± 10 | 13 ± 2 | 456 | NP |
| CS/HA35 | 35 | 72 ± 5 | 4.0 ± 0.5 | 100 ± 15 | 16 ± 3 | 432 | NP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestro, I.; Lopreiato, M.; Scotto d’Abusco, A.; Di Lisio, V.; Martinelli, A.; Piozzi, A.; Francolini, I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. Int. J. Mol. Sci. 2020, 21, 2070. https://doi.org/10.3390/ijms21062070
Silvestro I, Lopreiato M, Scotto d’Abusco A, Di Lisio V, Martinelli A, Piozzi A, Francolini I. Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. International Journal of Molecular Sciences. 2020; 21(6):2070. https://doi.org/10.3390/ijms21062070
Chicago/Turabian StyleSilvestro, Ilaria, Mariangela Lopreiato, Anna Scotto d’Abusco, Valerio Di Lisio, Andrea Martinelli, Antonella Piozzi, and Iolanda Francolini. 2020. "Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings" International Journal of Molecular Sciences 21, no. 6: 2070. https://doi.org/10.3390/ijms21062070
APA StyleSilvestro, I., Lopreiato, M., Scotto d’Abusco, A., Di Lisio, V., Martinelli, A., Piozzi, A., & Francolini, I. (2020). Hyaluronic Acid Reduces Bacterial Fouling and Promotes Fibroblasts’ Adhesion onto Chitosan 2D-Wound Dressings. International Journal of Molecular Sciences, 21(6), 2070. https://doi.org/10.3390/ijms21062070