The Role of Taste Receptor mTAS1R3 in Chemical Communication of Gametes
Abstract
:1. Introduction
2. Results
2.1. The Analysis of mRNA Expression of Tas1r3 Gene in Mouse Tissue
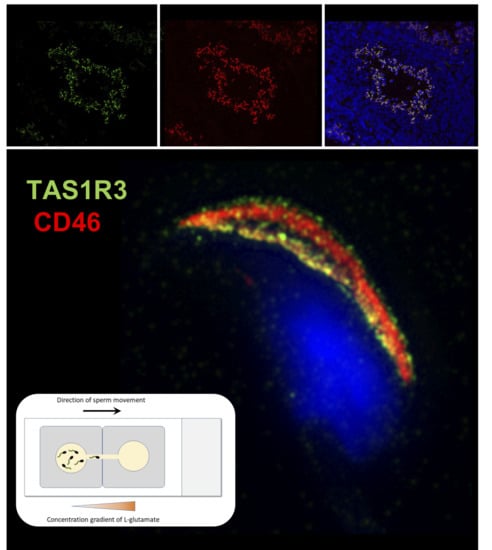
2.2. Localization of mTAS1R3 During Sperm Maturation
2.3. Sperm Chemotactic Responsiveness to L-glutamate
3. Discussion
4. Material and Methods
4.1. Animals
4.2. qPCR
4.3. Mouse Testes Cryosection Preparation
4.4. Sperm Sample Collection
4.5. Sperm Capacitation and Acrosome Reaction
4.6. Immunofluorescent Detection of mTAS1R3 with Confocal and Structured illumination Microscopy (SIM)
4.7. Sperm Chemotactic Responsiveness to L-glutamate
4.8. Sperm Chemotactic Response to L-glutamate in Presence of Antibody
4.9. Sperm Chemotactic Response COC in Presence of Antibody
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eisenbach, M.; Giojalas, L.C. Sperm guidance in mammals—An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006, 7, 276–285. [Google Scholar] [CrossRef]
- Perez-Cerezales, S.; Boryshpolets, S.; Eisenbach, M. Behavioral mechanisms of mammalian sperm guidance. Asian J. Androl. 2015, 17, 628–632. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Teves, M.E.; Unates, D.R.; Giojalas, L.C. Sperm transport and retention at the fertilization site is orchestrated by a chemical guidance and oviduct movement. Reproduction 2012, 143, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Harper, M.J. Stimulation of sperm movement from the isthmus to the site of fertilization in the rabbit oviduct. Biol. Reprod. 1973, 8, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Luddi, A.; Governini, L.; Wilmskotter, D.; Gudermann, T.; Boekhoff, I.; Piomboni, P. Taste Receptors: New Players in Sperm Biology. Int. J. Mol. Sci. 2019, 20, 967. [Google Scholar] [CrossRef] [Green Version]
- Simons, J.; Fauci, L. A Model for the Acrosome Reaction in Mammalian Sperm. Bull. Math. Biol. 2018, 80, 2481–2501. [Google Scholar] [CrossRef]
- Clift, L.E.; Andrlikova, P.; Frolikova, M.; Stopka, P.; Bryja, J.; Flanagan, B.F.; Johnson, P.M.; Dvorakova-Hortova, K. Absence of spermatozoal CD46 protein expression and associated rapid acrosome reaction rate in striped field mice (Apodemus agrarius). Reprod. Biol. Endocrinol. 2009, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.M.; Clift, L.E.; Andrlikova, P.; Jursova, M.; Flanagan, B.F.; Cummerson, J.A.; Stopka, P.; Dvorakova-Hortova, K. Rapid sperm acrosome reaction in the absence of acrosomal CD46 expression in promiscuous field mice (Apodemus). Reproduction 2007, 134, 739–747. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [Green Version]
- Meyer, D.; Voigt, A.; Widmayer, P.; Borth, H.; Huebner, S.; Breit, A.; Marschall, S.; De Angelis, M.H.; Boehm, U.; Meyerhof, W.; et al. Expression of Tas1 taste receptors in mammalian spermatozoa: Functional role of Tas1r1 in regulating basal Ca(2)(+) and cAMP concentrations in spermatozoa. PLoS ONE 2012, 7, e32354. [Google Scholar] [CrossRef] [Green Version]
- Hino, T.; Muro, Y.; Tamura-Nakano, M.; Okabe, M.; Tateno, H.; Yanagimachi, R. The Behavior and Acrosomal Status of Mouse Spermatozoa In Vitro, and Within the Oviduct During Fertilization after Natural Mating. Biol. Reprod. 2016, 95, 50. [Google Scholar] [CrossRef]
- La Spina, F.A.; Puga Molina, L.C.; Romarowski, A.; Vitale, A.M.; Falzone, T.L.; Krapf, D.; Hirohashi, N.; Buffone, M.G. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 2016, 411, 172–182. [Google Scholar] [CrossRef]
- Muro, Y.; Hasuwa, H.; Isotani, A.; Miyata, H.; Yamagata, K.; Ikawa, M.; Yanagimachi, R.; Okabe, M. Behavior of Mouse Spermatozoa in the Female Reproductive Tract from Soon after Mating to the Beginning of Fertilization. Biol. Reprod. 2016, 94, 80. [Google Scholar] [CrossRef]
- Hoon, M.A.; Adler, E.; Lindemeier, J.; Battey, J.F.; Ryba, N.J.; Zuker, C.S. Putative mammalian taste receptors: A class of taste-specific GPCRs with distinct topographic selectivity. Cell 1999, 96, 541–551. [Google Scholar] [CrossRef] [Green Version]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Zhang, J.; Yang, H.; Zhang, Y.P. Adaptive diversification of bitter taste receptor genes in Mammalian evolution. Mol. Biol. Evol. 2003, 20, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Meyerhof, W.; Hellfritsch, C.; Hofmann, T. Sweet and umami taste: Natural products, their chemosensory targets, and beyond. Angew. Chem. Int. Ed. Engl. 2011, 50, 2220–2242. [Google Scholar] [CrossRef]
- Li, F.; Zhou, M. Depletion of bitter taste transduction leads to massive spermatid loss in transgenic mice. Mol. Hum. Reprod. 2012, 18, 289–297. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Nomura, M.; Shibata, A.; Ichikawa, R.; Enciso, P.L.; Wang, L.; Takayanagi, R.; Torii, K.; Uneyama, H. Generation and characterization of T1R2-LacZ knock-in mouse. Biochem. Biophys. Res. Commun. 2010, 402, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Taste perception: From the tongue to the testis. Mol. Hum. Reprod. 2013, 19, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.E.; Gopichandran, N.; Picton, H.M.; Leese, H.J.; Orsi, N.M. Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology 2005, 64, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Brandis, A.; Nevo, R.; Kiss, V.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5, 16146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopková, R.; Vinkler, D.; Kuntová, B.; Šedo, O.; Albrecht, T.; Suchan, J.; Dvořáková-Hortová, K.; Zdráhal, Z.; Stopka, P. Mouse Lipocalins (MUP, OBP, LCN) Are Co-expressed in Tissues Involved in Chemical Communication. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Mombaerts, P. Molecular biology of odorant receptors in vertebrates. Annu. Rev. Neurosci. 1999, 22, 487–509. [Google Scholar] [CrossRef] [PubMed]
- Frolíková, M.; Stopková, R.; Antalíková, J.; Johnson, P.M.; Stopka, P.; Dvořáková-Hortová, K. Role of complement regulatory proteins CD46, CD55 and CD59 in reproduction. Folia Zool. 2012, 61, 84–94. [Google Scholar] [CrossRef]
- Frolikova, M.; Sebkova, N.; Ded, L.; Dvorakova-Hortova, K. Characterization of CD46 and beta1 integrin dynamics during sperm acrosome reaction. Sci. Rep. 2016, 6, 33714. [Google Scholar] [CrossRef] [Green Version]
- Inoue, N.; Ikawa, M.; Nakanishi, T.; Matsumoto, M.; Nomura, M.; Seya, T.; Okabe, M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell Biol. 2003, 23, 2614–2622. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.; Ikawa, M.; Yamada, S.; Parvinen, M.; Baba, T.; Nishimune, Y.; Okabe, M. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999, 449, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Fremeau, R.T., Jr.; Troyer, M.D.; Pahner, I.; Nygaard, G.O.; Tran, C.H.; Reimer, R.J.; Bellocchio, E.E.; Fortin, D.; Storm-Mathisen, J.; Edwards, R.H. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 2001, 31, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Juge, N.; Gray, J.A.; Omote, H.; Miyaji, T.; Inoue, T.; Hara, C.; Uneyama, H.; Edwards, R.H.; Nicoll, R.A.; Moriyama, Y. Metabolic control of vesicular glutamate transport and release. Neuron 2010, 68, 99–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.H.; Yang, N.; Ma, Y.H.; Jiang, J.; Zhang, J.F.; Fei, J.; Guo, L.H. Identification of glutamate receptors and transporters in mouse and human sperm. J. Androl. 2004, 25, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Zitranski, N.; Borth, H.; Ackermann, F.; Meyer, D.; Vieweg, L.; Breit, A.; Gudermann, T.; Boekhoff, I. The “acrosomal synapse”: Subcellular organization by lipid rafts and scaffolding proteins exhibits high similarities in neurons and mammalian spermatozoa. Commun. Integr. Biol. 2010, 3, 513–521. [Google Scholar] [CrossRef]
- Spehr, M.; Gisselmann, G.; Poplawski, A.; Riffell, J.A.; Wetzel, C.H.; Zimmer, R.K.; Hatt, H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 2003, 299, 2054–2058. [Google Scholar] [CrossRef]
- Flegel, C.; Vogel, F.; Hofreuter, A.; Schreiner, B.S.; Osthold, S.; Veitinger, S.; Becker, C.; Brockmeyer, N.H.; Muschol, M.; Wennemuth, G.; et al. Characterization of the Olfactory Receptors Expressed in Human Spermatozoa. Front. Mol. Biosci. 2015, 2, 73. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Yoshida, K. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 2011, 17, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Jozwik, M.; Jozwik, M.; Teng, C.; Battaglia, F.C. Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid. Hum. Reprod. 2006, 21, 2776–2782. [Google Scholar] [CrossRef]
- Uhde, K.; van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci. Rep. 2018, 8, 9477. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Hirohashi, N.; Cubilla, M.; Buffone, M.G.; Giojalas, L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017, 84, 310–315. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebkova, N.; Ded, L.; Vesela, K.; Dvorakova-Hortova, K. Progress of sperm IZUMO1 relocation during spontaneous acrosome reaction. Reproduction 2014, 147, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, D.; Kon, S.; Sato, T.; Toyama, F.; Katsura, Y.; Nakauchi, Y.; Takayama-Watanabe, E.; Watanabe, A. NMDA-type glutamate receptors mediate the acrosome reaction and motility initiation in newt sperm. Mol. Reprod. Dev. 2019, 86, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Storto, M.; Sallese, M.; Salvatore, L.; Poulet, R.; Condorelli, D.F.; Dell’Albani, P.; Marcello, M.F.; Romeo, R.; Piomboni, P.; Barone, N.; et al. Expression of metabotropic glutamate receptors in the rat and human testis. J. Endocrinol. 2001, 170, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, N.; Landin, A.M.; Roper, S.D. A metabotropic glutamate receptor variant functions as a taste receptor. Nat. Neurosci. 2000, 3, 113–119. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frolikova, M.; Otcenaskova, T.; Valasková, E.; Postlerova, P.; Stopkova, R.; Stopka, P.; Komrskova, K. The Role of Taste Receptor mTAS1R3 in Chemical Communication of Gametes. Int. J. Mol. Sci. 2020, 21, 2651. https://doi.org/10.3390/ijms21072651
Frolikova M, Otcenaskova T, Valasková E, Postlerova P, Stopkova R, Stopka P, Komrskova K. The Role of Taste Receptor mTAS1R3 in Chemical Communication of Gametes. International Journal of Molecular Sciences. 2020; 21(7):2651. https://doi.org/10.3390/ijms21072651
Chicago/Turabian StyleFrolikova, Michaela, Tereza Otcenaskova, Eliska Valasková, Pavla Postlerova, Romana Stopkova, Pavel Stopka, and Katerina Komrskova. 2020. "The Role of Taste Receptor mTAS1R3 in Chemical Communication of Gametes" International Journal of Molecular Sciences 21, no. 7: 2651. https://doi.org/10.3390/ijms21072651
APA StyleFrolikova, M., Otcenaskova, T., Valasková, E., Postlerova, P., Stopkova, R., Stopka, P., & Komrskova, K. (2020). The Role of Taste Receptor mTAS1R3 in Chemical Communication of Gametes. International Journal of Molecular Sciences, 21(7), 2651. https://doi.org/10.3390/ijms21072651