Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration
Abstract
:1. Introduction
2. Results
2.1. The Effects of BMPs on Degenerated Human NP Cells
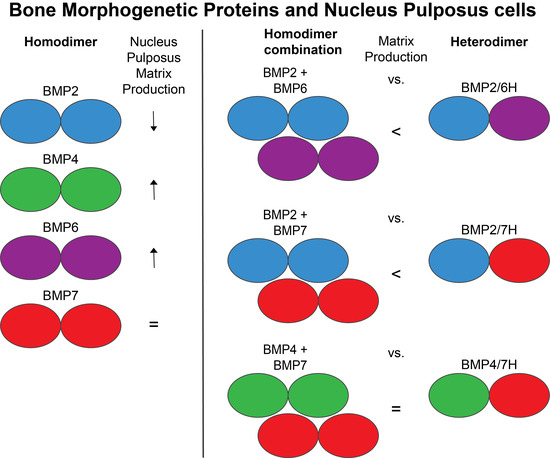
2.1.1. GAG Deposition
2.1.2. Collagen Production and Deposition
2.1.3. Dose Dependency of BMP4 and BMP7 Stimulation
2.2. Pilot Study: The Effects of BMPs on Co-Cultures of NP Cells and MSCs
2.2.1. MSC Multipotency
2.2.2. BMPs with NP Cells Co-Cultured with MSCs
3. Discussion
4. Materials and Methods
4.1. Cultures
4.1.1. Cell Isolation
4.1.2. BMPs in NP Differentiation Culture
4.1.3. MSC Multipotency Assays
4.1.4. MSC and NP Cell Co-Culture
4.2. Analyses
4.2.1. Biochemistry: Tissue GAG and DNA Content
4.2.2. Procollagen II ELISA
4.2.3. Histology
4.2.4. Immunohistochemistry
4.3. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| ASAP | ascorbic acid-2-phosphate |
| bFGF | human basic fibroblast growth factor |
| BMP | bone morphogenetic protein |
| BSA | bovine serum albumin |
| α-MEM | Eagle’s minimum essential medium – alpha modification |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMMB | 1,9-dimethylmethylene blue |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| GAG | glycosaminoglycan |
| H | heterodimers |
| IBMX | 3-isobutyl-1-methylxanthine |
| ITS | insulin-transferrin-selenium |
| IVD | intervertebral disc |
| METC | medical ethics committee |
| MMP | matrix metalloprotease |
| MSC | mesenchymal stromal cell |
| NCT | national clinical trial |
| NP | nucleus pulposus |
| OD | optical density |
| pen/strep | penicillin + streptomycin |
| PIICP | carboxy-terminal propeptide of type II collagen or procollagen II |
| TGF-β | transforming growth factor-β |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| SD | standard deviation |
References
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Luoma, K.; Riihimaki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A. Low back pain in relation to lumbar disc degeneration. Spine 2000, 25, 487–492. [Google Scholar] [CrossRef]
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2015, 36, 2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. 2003, 5, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Kennon, J.C.; Awad, M.E.; Chutkan, N.; DeVine, J.; Fulzele, S. Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomol. Concepts 2018, 9, 43–52. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Luca, F.D.; Barnes, K.M.; Uyeda, J.A.; De-Levi, S.; Abad, V.; Palese, T.; Mericq, V.; Baron, J. Regulation of Growth Plate Chondrogenesis by Bone Morphogenetic Protein-2. Endocrinology 2001, 142, 430–436. [Google Scholar] [CrossRef]
- Pregizer, S.K.; Mortlock, D.P. Dynamics and Cellular Localization of Bmp2, Bmp4, and Noggin Transcription in the Postnatal Mouse Skeleton. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Le Douarin, N.M. A role for BMP-4 in the development of subcutaneous cartilage. Mech. Dev. 1996, 57, 69–78. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Tuan, R.S.; Shum, L. Distinct functions of BMP4 and GDF5 in the regulation of chondrogenesis. J. Cell. Biochem. 2004, 91, 1204–1217. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Boskey, A.L.; Paschalis, E.P.; Binderman, I.; Doty, S.B. BMP-6 accelerates both chondrogenesis and mineral maturation in differentiating chick limb-bud mesenchymal cell cultures. J. Cell. Biochem. 2002, 84, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Grimsrud, C.D.; Romano, P.R.; D’Souza, M.; Puzas, J.E.; Reynolds, P.R.; Rosier, R.N.; O’Keefe, R.J. BMP-6 Is an Autocrine Stimulator of Chondrocyte Differentiation. J. Bone Miner. Res. 1999, 14, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chubinskaya, S.; Hurtig, M.; Rueger, D. OP-1/BMP-7 in cartilage repair. Int. Orthop. 2007, 31, 773–781. [Google Scholar] [CrossRef]
- Aono, A.; Hazama, M.; Notoya, K.; Taketomi, S.; Yamasaki, H.; Tsukuda, R.; Sasaki, S.; Fujisawa, Y. Potent Ectopic Bone-Inducing Activity of Bone Morphogenetic Protein-4/7 Heterodimer. Biochem. Biophys. Res. Commun. 1995, 210, 670–677. [Google Scholar] [CrossRef]
- Israel, D.I.; Nove, J.; Kerns, K.M.; Kaufman, R.J.; Rosen, V.; Cox, K.A.; Wozney, J.M. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 1996, 13, 291–300. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.; Zheng, Y.; Fan, Y.; Gu, Z. BMP2/7 heterodimer is a stronger inducer of bone regeneration in peri-implant bone defects model than BMP2 or BMP7 homodimer. Dent. Mater. J. 2012, 31, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Loozen, L.D.; Vandersteen, A.; Kragten, A.H.; Oner, F.C.; Dhert, W.J.; Kruyt, M.C.; Alblas, J. Bone formation by heterodimers through non-viral gene delivery of BMP-2/6 and BMP-2/7. Eur. Cells Mater. 2018, 35, 195–208. [Google Scholar] [CrossRef]
- Miao, C.; Qin, D.; Cao, P.; Lu, P.; Xia, Y.; Li, M.; Sun, M.; Zhang, W.; Yang, F.; Zhang, Y.; et al. BMP2/7 heterodimer enhances osteogenic differentiation of rat BMSCs via ERK signaling compared with respective homodimers. J. Cell. Biochem. 2018, 120, 8754–8763. [Google Scholar] [CrossRef]
- Li, Z.; Lang, G.; Karfeld-Sulzer, L.S.; Mader, K.T.; Richards, R.G.; Weber, F.E.; Sammon, C.; Sacks, H.; Yayon, A.; Alini, M.; et al. Heterodimeric BMP-2/7 for nucleus pulposus regeneration—In vitro and ex vivo studies. J. Orthop. Res. 2017, 35, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Pan, W.; Hu, W.; Chen, L. Bone morphogenetic protein-7 retards cell subculture-induced senescence of human nucleus pulposus cells through activating the PI3K/Akt pathway. Biosci. Rep. 2019, 39, BSR20182312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Yao, X.; Dai, Z.; Wang, Y.; Lv, G. Bone morphogenetic protein 2 alleviated intervertebral disc degeneration through mediating the degradation of ECM and apoptosis of nucleus pulposus cells via the PI3K/Akt pathway. Int. J. Mol. Med. 2019, 43, 583–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, S.D. The ABCs of BMPs. Orthop. Nurs. 2005, 24, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, M.; Hashizume, H.; Matsumoto, T.; Enyo, Y.; Okada, M.; Yoshida, M.; Chubinskaya, S. Safety of epidural administration of Osteogenic Protein-1 (OP-1/BMP-7): Behavioral and macroscopic observation. Spine 2007, 32, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- An, H.S.; Takegami, K.; Kamada, H.; Nguyen, C.M.; Thonar, E.J.; Singh, K.; Andersson, G.B.; Masuda, K. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 2005, 30, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Imai, Y.; Okuma, M.; Muehleman, C.; Nakagawa, K.; Akeda, K.; Thonar, E.; Andersson, G.; An, H.S. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine 2006, 31, 742–754. [Google Scholar] [CrossRef]
- Willems, N.; Bach, F.C.; Plomp, S.G.M.; van Rijen, M.H.P.; Wolfswinkel, J.; Grinwis, G.C.M.; Bos, C.; Strijkers, G.J.; Dhert, W.J.A.; Meij, B.P.; et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 2015, 17, 137. [Google Scholar] [CrossRef] [Green Version]
- Rudnik-Jansen, I.; Tellegen, A.; Beukers, M.; Öner, F.; Woike, N.; Mihov, G.; Thies, J.; Meij, B.; Tryfonidou, M.; Creemers, L. Safety of intradiscal delivery of triamcinolone acetonide by a poly(esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 2019, 19, 905–919. [Google Scholar] [CrossRef]
- Allon, A.A.; Butcher, K.; Schneider, R.A.; Lotz, J.C. Structured coculture of mesenchymal stem cells and disc cells enhances differentiation and proliferation. Cells Tissues Organs 2012, 196, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Le Visage, C.; Kim, S.W.; Tateno, K.; Sieber, A.N.; Kostuik, J.P.; Leong, K.W. Interaction of Human Mesenchymal Stem Cells With Disc Cells: Changes in Extracellular Matrix Biosynthesis. Spine 2006, 31, 2036–2042. [Google Scholar] [CrossRef]
- Sobajima, S.; Vadala, G.; Shimer, A.; Kim, J.S.; Gilbertson, L.G.; Kang, J.D. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008, 8, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Svanvik, T.; Barreto Henriksson, H.; Karlsson, C.; Hagman, M.; Lindahl, A.; Brisby, H. Human Disk Cells from Degenerated Disks and Mesenchymal Stem Cells in Co-Culture Result in Increased Matrix Production. Cells Tissues Organs 2010, 191, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Studer, R.K.; Sowa, G.; Spiezia, F.; Iucu, C.; Denaro, V.; Gilbertson, L.G.; Kang, J.D. Coculture of Bone Marrow Mesenchymal Stem Cells and Nucleus Pulposus Cells Modulate Gene Expression Profile Without Cell Fusion. Spine 2008, 33, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; E, X.-Q.; Wang, N.-X.; Wang, M.-N.; Xie, H.-X.; Cao, Y.-H.; Sun, L.-H.; Tian, J.; Chen, H.-J.; Yan, J.-L. BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration. FEBS J. 2016, 283, 1689–1700. [Google Scholar] [CrossRef] [Green Version]
- Bach, F.C.; Miranda-Bedate, A.; van Heel, F.W.M.; Riemers, F.M.; Müller, M.C.M.E.; Creemers, L.B.; Ito, K.; Benz, K.; Meij, B.P.; Tryfonidou, M.A. Bone Morphogenetic Protein-2, But Not Mesenchymal Stromal Cells, Exert Regenerative Effects on Canine and Human Nucleus Pulposus Cells. Tissue Eng. Part A 2016, 23, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Than, K.D.; Rahman, S.U.; Vanaman, M.J.; Wang, A.C.; Lin, C.-Y.; Zhang, H.; La Marca, F.; Park, P. Bone Morphogenetic Proteins and Degenerative Disk Disease. Neurosurgery 2012, 70, 996–1002. [Google Scholar] [CrossRef]
- Leung, V.Y.L.; Zhou, L.; Tam, W.-K.; Sun, Y.; Lv, F.; Zhou, G.; Cheung, K.M.C. Bone morphogenetic protein-2 and -7 mediate the anabolic function of nucleus pulposus cells with discrete mechanisms. Connect. Tissue Res. 2017, 58, 573–585. [Google Scholar] [CrossRef]
- Leckie, S.K.; Bechara, B.P.; Hartman, R.A.; Sowa, G.A.; Woods, B.I.; Coelho, J.P.; Witt, W.T.; Dong, Q.D.; Bowman, B.W.; Bell, K.M.; et al. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012, 12, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Chen, L.K.; Zhu, D.C.; Zhang, G.R.; Guo, C.; Qi, Y.Y.; Ouyang, H.W. The Inductive Effect of Bone Morphogenetic Protein-4 on Chondral-Lineage Differentiation and In Situ Cartilage Repair. Tissue Eng. Part A 2009, 16, 1621–1632. [Google Scholar] [CrossRef]
- Zhang, Y.; An, H.S.; Thonar, E.J.-M.A.; Chubinskaya, S.; He, T.-C.; Phillips, F.M. Comparative Effects of Bone Morphogenetic Proteins and Sox9 Overexpression on Extracellular Matrix Metabolism of Bovine Nucleus Pulposus Cells. Spine 2006, 31, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- Alini, M.; Eisenstein, S.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavery, K.; Swain, P.; Falb, D.; Alaoui-Ismaili, M.H. BMP-2/4 and BMP-6/7 Differentially Utilize Cell Surface Receptors to Induce Osteoblastic Differentiation of Human Bone Marrow-derived Mesenchymal Stem Cells. J. Biol. Chem. 2008, 283, 20948–20958. [Google Scholar] [CrossRef] [Green Version]
- Valera, E.; Isaacs, M.J.; Kawakami, Y.; Izpisúa Belmonte, J.C.; Choe, S. BMP-2/6 Heterodimer Is More Effective than BMP-2 or BMP-6 Homodimers as Inductor of Differentiation of Human Embryonic Stem Cells. PLoS ONE 2010, 5, e11167. [Google Scholar] [CrossRef] [Green Version]
- Tillet, E.; Ouarné, M.; Desroches-Castan, A.; Mallet, C.; Subileau, M.; Didier, R.; Lioutsko, A.; Belthier, G.; Feige, J.-J.; Bailly, S. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J. Biol. Chem. 2018, 293, 10963–10974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krase, A.; Abedian, R.; Steck, E.; Hurschler, C.; Richter, W. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthr. Cartil. 2014, 22, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, M.J.; Kawakami, Y.; Allendorph, G.P.; Yoon, B.-H.; Belmonte, J.C.I.; Choe, S. Bone Morphogenetic Protein-2 and -6 Heterodimer Illustrates the Nature of Ligand-Receptor Assembly. Mol. Endocrinol. 2010, 24, 1469–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neugebauer, J.M.; Kwon, S.; Kim, H.-S.; Donley, N.; Tilak, A.; Sopory, S.; Christian, J.L. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, E2307–E2316. [Google Scholar] [CrossRef] [Green Version]
- Little, S.C.; Mullins, M.C. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat. Cell Biol. 2009, 11, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Kim, J.; Cheng, C.; Rawlins, B.A.; Boachie-Adjei, O.; Crystal, R.G.; Hidaka, C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 2006, 39, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Shimmi, O.; Umulis, D.; Othmer, H.; O’Connor, M.B. Facilitated Transport of a Dpp/Scw Heterodimer by Sog/Tsg Leads to Robust Patterning of the Drosophila Blastoderm Embryo. Cell 2005, 120, 873–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberstroh, K.; Enz, A.; Zenclussen, M.L.; Hegewald, A.A.; Neumann, K.; Abbushi, A.; Thomé, C.; Sittinger, M.; Endres, M.; Kaps, C. Human intervertebral disc-derived cells are recruited by human serum and form nucleus pulposus-like tissue upon stimulation with TGF-β3 or hyaluronan in vitro. Tissue Cell 2009, 41, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yoon, Y.S.; Park, H.S.; Kuh, S.U. Molecular response of human cervical and lumbar nucleus pulposus cells from degenerated discs following cytokine treatment. Genet Mol. Res. 2013, 12, 838–851. [Google Scholar] [CrossRef]
- Yang, S.-H.; Yang, K.-C.; Chen, C.-W.; Huang, T.-C.; Sun, Y.-H.; Hu, M.-H. Comparison of Transforming Growth Factor-Beta1 and Lovastatin on Differentiating Mesenchymal Stem Cells toward Nucleus Pulposus-like Phenotype: An In Vitro Cell Culture Study. Asian Spine J. 2019, 13, 705–712. [Google Scholar] [CrossRef]
- Jin, E.S.; Min, J.; Jeon, S.R.; Choi, K.H.; Jeong, J.H. Analysis of molecular expression in adipose tissue-derived mesenchymal stem cells: Prospects for use in the treatment of intervertebral disc degeneration. J. Korean Neurosurg. Soc. 2013, 53, 207–212. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, G.; Zhang, L.; Hu, Y.; Zhang, K.; Sun, X.; Zhao, C.; Li, H.; Li, Y.M.; Zhao, J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J. Cell Mol. Med. 2018, 22, 261–276. [Google Scholar] [CrossRef]
- Miyamoto, T.; Muneta, T.; Tabuchi, T.; Matsumoto, K.; Saito, H.; Tsuji, K.; Sekiya, I. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res. Ther. 2010, 12, R206. [Google Scholar] [CrossRef] [Green Version]
- Vaudreuil, N.; Henrikson, K.; Pohl, P.; Lee, A.; Lin, H.; Olsen, A.; Dong, Q.; Dombrowski, M.; Kang, J.; Vo, N.; et al. Photopolymerizable biogel scaffold seeded with mesenchymal stem cells: Safety and efficacy evaluation of novel treatment for intervertebral disc degeneration. J. Orthop. Res. 2019, 37, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.; Suzuki, R.; Sand, T.; Murphy, M. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. Int. Orthop. 2015, 40, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Ha, D.H.; Lee, E.J.; Park, J.H.; Shim, J.H.; Ahn, T.K.; Kim, K.T.; Ropper, A.E.; Sohn, S.; Kim, C.H.; et al. Safety and tolerability of intradiscal implantation of combined autologous adipose-derived mesenchymal stem cells and hyaluronic acid in patients with chronic discogenic low back pain: 1-year follow-up of a phase I study. Stem Cell Res. Ther. 2017, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Strassburg, S.; Richardson, S.M.; Freemont, A.J.; Hoyland, J.A. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen. Med. 2010, 5, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Deng, G.; Tian, Y.; Pu, Y.; Cao, P.; Yuan, W. An in vitro investigation into the role of bone marrowderived mesenchymal stem cells in the control of disc degeneration. Mol. Med. Rep. 2015, 12, 5701–5708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Windt, T.S.; Saris, D.B.F.; Slaper-Cortenbach, I.C.M.; van Rijen, M.H.P.; Gawlitta, D.; Creemers, L.B.; de Weger, R.A.; Dhert, W.J.A.; Vonk, L.A. Direct Cell–Cell Contact with Chondrocytes Is a Key Mechanism in Multipotent Mesenchymal Stromal Cell-Mediated Chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Prins, H.J.; Helder, M.N.; van Blitterswijk, C.A.; Karperien, M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng. Part A 2012, 18, 1542–1551. [Google Scholar] [CrossRef]
- Fontana, G.; See, E.; Pandit, A. Current trends in biologics delivery to restore intervertebral disc anabolism. Adv. Drug Deliv. Rev. 2014, 84, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-W.; Liu, K.; Chen, Z.; Zhao, M.; Han, X.-W.; Bai, Y.-G.; Feng, G. Adenovirus-mediated GDF-5 promotes the extracellular matrix expression in degenerative nucleus pulposus cells. J. Zhejiang Univ. Sci. B 2016, 17, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int. J. Exp. Pathol. 2006, 87, 17–28. [Google Scholar] [CrossRef]
- Morrey, M.E.; Anderson, P.A.; Chambers, G.; Paul, R. Optimizing nonviral-mediated transfection of human intervertebral disc chondrocytes. Spine J. 2008, 8, 796–803. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, K.; Liu, G.; Ding, W.; Zhao, C.; Xie, Y.; Yuan, J.; Sun, X.; Li, H.; Liu, C.; et al. Sox9 Gene Transfer Enhanced Regenerative Effect of Bone Marrow Mesenchymal Stem Cells on the Degenerated Intervertebral Disc in a Rabbit Model. PLoS ONE 2014, 9, e93570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, H.; Zilberman, Y.; Arbeli, V.; Sheyn, D.; Matan, Y.; Liebergall, M.; Li, J.Z.; Helm, G.A.; Gazit, D.; Gazit, Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006, 12, 877–889. [Google Scholar] [CrossRef]
- Sheyn, D.; Pelled, G.; Zilberman, Y.; Talasazan, F.; Frank, J.M.; Gazit, D.; Gazit, Z. Nonvirally Engineered Porcine Adipose Tissue-Derived Stem Cells: Use in Posterior Spinal Fusion. Stem Cells 2008, 26, 1056–1064. [Google Scholar] [CrossRef] [Green Version]
- Hingert, D.; Barreto Henriksson, H.; Brisby, H. Human Mesenchymal Stem Cells Pretreated with Interleukin-1beta and Stimulated with Bone Morphogenetic Growth Factor-3 Enhance Chondrogenesis. Tissue Eng. Part A 2018, 24, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.P.; Pearce, R.H.; Schechter, M.T.; Adams, M.E.; Tsang, I.K.Y.; Bishop, P.B. Preliminary Evaluation of a Scheme for Grading the Gross Morphology of the Human Intervertebral Disc. Spine 1990, 15, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Nelson, F.; Dahlberg, L.; Laverty, S.; Reiner, A.; Pidoux, I.; Ionescu, M.; Fraser, G.L.; Brooks, E.; Tanzer, M.; Rosenberg, L.C.; et al. Evidence for altered synthesis of type II collagen in patients with osteoarthritis. J. Clin. Investig. 1998, 102, 2115–2125. [Google Scholar] [CrossRef] [Green Version]
- Krouwels, A.; Popov-Celeketic, J.; Plomp, S.G.M.; Dhert, W.J.A.; Öner, F.C.; Bank, R.A.; Creemers, L.B. No Effects of Hyperosmolar Culture Medium on Tissue Regeneration by Human Degenerated Nucleus Pulposus Cells Despite Upregulation Extracellular Matrix Genes. Spine 2018, 43, 307–315. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krouwels, A.; Iljas, J.D.; Kragten, A.H.M.; Dhert, W.J.A.; Öner, F.C.; Tryfonidou, M.A.; Creemers, L.B. Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration. Int. J. Mol. Sci. 2020, 21, 2720. https://doi.org/10.3390/ijms21082720
Krouwels A, Iljas JD, Kragten AHM, Dhert WJA, Öner FC, Tryfonidou MA, Creemers LB. Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration. International Journal of Molecular Sciences. 2020; 21(8):2720. https://doi.org/10.3390/ijms21082720
Chicago/Turabian StyleKrouwels, Anita, Juvita D. Iljas, Angela H. M. Kragten, Wouter J. A. Dhert, F. Cumhur Öner, Marianna A. Tryfonidou, and Laura B. Creemers. 2020. "Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration" International Journal of Molecular Sciences 21, no. 8: 2720. https://doi.org/10.3390/ijms21082720
APA StyleKrouwels, A., Iljas, J. D., Kragten, A. H. M., Dhert, W. J. A., Öner, F. C., Tryfonidou, M. A., & Creemers, L. B. (2020). Bone Morphogenetic Proteins for Nucleus Pulposus Regeneration. International Journal of Molecular Sciences, 21(8), 2720. https://doi.org/10.3390/ijms21082720