Tolerance of Facultative Metallophyte Carlina acaulis to Cadmium Relies on Chelating and Antioxidative Metabolites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of Chronic/Acute Cd Stress on the Growth
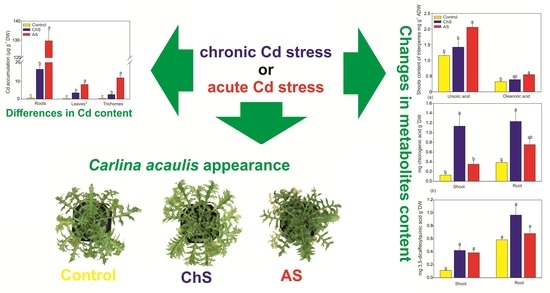
2.2. Accumulation of Cd and Mineral. Nutrients
2.3. Changes in Antioxidants and Chelators Differ. under Acute and Chronic Cd Stress
2.4. Changes of Secondary Metabolites under Various Exposure
2.5. Principle Component Analysis
3. Materials and Methods
3.1. Plant. Material, Growth Conditions, and Experimental Design
3.2. Determination of Cd and Mineral. Nutrients
3.3. Measurement of Organic Acids, Ascorbic Acid, and Thiols
3.4. HPLC of Triterpene and Phenolic Acids
3.5. Quantification of Total Phenolic Content and Antioxidant Capacity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanità Di Toppi, L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef]
- Arduini, I.; Masoni, A.; Mariotti, M.; Ercoli, L. Low cadmium application increase miscanthus growth and cadmium translocation. Environ. Exp. Bot. 2004, 52, 89–100. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Štork, F.; Hedbavny, J. Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application. J. Hazard. Mater. 2012, 239–240, 175–182. [Google Scholar] [CrossRef]
- Andresen, E.; Kappel, S.; Stärk, H.-J.; Riegger, U.; Borovec, J.; Mattusch, J.; Heinz, A.; Schmelzer, C.E.H.; Matoušková, Š.; Dickinson, B.; et al. Cadmium toxicity investigated at the physiological and biophysical levels under environmentally relevant conditions using the aquatic model plant Ceratophyllum demersum. New Phytol. 2016, 210, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Mattusch, J.; Wellenreuther, G.; Thomas, G.; Abad, U.A.; Küpper, H. Different strategies of cadmium detoxification in the submerged macrophyte Ceratophyllum demersum L. Metallomics 2013, 5, 1377–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perreault, F.; Dionne, J.; Didur, O.; Juneau, P.; Popovic, R. Effect of cadmium on photosystem II activity in Chlamydomonas reinhardtii: Alteration of O–J–I–P fluorescence transients indicating the change of apparent activation energies within photosystem II. Photosynth. Res. 2011, 107, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B. Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant. Cell Rep. 2008, 27, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Bujdoš, M.; Ketzer, P.; Babula, P.; Peterková, V.; Krenn, L. Dandelion is more tolerant to cadmium than to nickel excess. Chemosphere 2019, 224, 884–891. [Google Scholar] [CrossRef]
- Kováčik, J. Role of low molecular weight compounds in cadmium stress tolerance. In Cadmium Tolerance in Plants; Hasanuzzaman, M., Vara Prasad, M.N., Nahar, K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 281–318. ISBN 978-0-12-815794-7. [Google Scholar]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Rutkowska, E.; Szwerc, W.; Kocjan, R.; Latalski, M. Carlina species as a new source of bioactive pentacyclic triterpenes. Ind. Crops Prod. 2016, 94, 498–504. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Agacka-Mołdoch, M.; Drączkowski, P.; Matosiuk, D.; Kurach, Ł.; Kocjan, R.; Dresler, S. Application of Raman spectroscopy for direct analysis of Carlina acanthifolia subsp. utzka root essential oil. Talanta 2017, 174, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and traditional medical applications of Carlina acaulis L.—A critical ethnopharmacological review. J. Ethnopharmacol. 2019. [Google Scholar] [CrossRef]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Szwerc, W.; Sawicki, J.; Kocjan, R.; Feldo, M.; Dresler, S. Carlina vulgaris L. as a source of phytochemicals with antioxidant activity. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jędrzejczyk-Korycińska, M. Floristic diversity in calamine areas of the Silesia-Cracow Monocline. Biodiv. Res. Conserv. 2006, 3–4, 340–343. [Google Scholar]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Larsson, E.H.; Bornman, J.F.; Asp, H. Influence of UV-B radiation and Cd2+ on chlorophyll fluorescence, growth and nutrient content in Brassica napus. J. Exp. Bot. 1998, 49, 1031–1039. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant. Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Calabrese, E.J. Evidence that hormesis represents an “overcompensation” response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef]
- Aina, R.; Labra, M.; Fumagalli, P.; Vannini, C.; Marsoni, M.; Cucchi, U.; Bracale, M.; Sgorbati, S.; Citterio, S. Thiol-peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environ. Exp. Bot. 2007, 59, 381–392. [Google Scholar] [CrossRef]
- Kováčik, J. Hyperaccumulation of cadmium in Matricaria chamomilla: A never-ending story? Acta Physiol. Plant. 2013, 35, 1721–1725. [Google Scholar] [CrossRef]
- Yu, H.; Guo, J.; Li, Q.; Zhang, X.; Huang, H.; Huang, F.; Yang, A.; Li, T. Characteristics of cadmium immobilization in the cell wall of root in a cadmium-safe rice line (Oryza sativa L.). Chemosphere 2020, 241, 125095. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in indian mustard. Plant. Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.-E.; Harada, E.; Wada, M.; Tsuboi, H.; Morita, Y.; Kusano, T.; Sano, H. Detoxification of cadmium in tobacco plants: Formation and active excretion of crystals containing cadmium and calcium through trichomes. Planta 2001, 213, 45–50. [Google Scholar] [CrossRef]
- Isaure, M.-P.; Fayard, B.; Sarret, G.; Pairis, S.; Bourguignon, J. Localization and chemical forms of cadmium in plant samples by combining analytical electron microscopy and X-ray spectromicroscopy. Spectrochim. Acta Part. B At. Spectrosc. 2006, 61, 1242–1252. [Google Scholar] [CrossRef] [Green Version]
- Hojati, M.; Modarres-Sanavy, S.A.M.; Enferadi, S.T.; Majdi, M.; Ghanati, F.; Farzadfar, S.; Pazoki, A. Cadmium and copper induced changes in growth, oxidative metabolism and terpenoids of Tanacetum parthenium. Environ. Sci. Pollut. Res. 2017, 24, 12261–12272. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Interaction between cadmium stress and sulphur nutrition level on macronutrient status of Sinapis alba L. Water Air Soil Pollut. 2016, 227, 355. [Google Scholar] [CrossRef] [Green Version]
- Nedjimi, B. Heavy metal tolerance in two Algerian saltbushes: A review on plant responses to cadmium and role of calcium in its mitigation. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer Singapore: Singapore, 2018; pp. 205–220. ISBN 978-981-10-9044-8. [Google Scholar]
- Kováčik, J.; Dresler, S. Calcium availability but not its content modulates metal toxicity in Scenedesmus quadricauda. Ecotoxicol. Environ. Saf. 2018, 147, 664–669. [Google Scholar] [CrossRef]
- Wang, C.Q.; Song, H. Calcium protects Trifolium repens L. seedlings against cadmium stress. Plant. Cell Rep. 2009, 28, 1341–1349. [Google Scholar] [CrossRef]
- Zenk, M.H. Heavy metal detoxification in higher plants-A review. Gene 1996, 179, 21–30. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Kováčik, J.; Pochwatka, M.; Hanaka, A.; Strzemski, M.; Sowa, I.; Wójciak-Kosior, M. Allantoin attenuates cadmium-induced toxicity in cucumber plants. Ecotoxicol. Environ. Saf. 2019, 170, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Babula, P.; Hedbavny, J. Ascorbic acid affects short-term response of Scenedesmus quadricauda to cadmium excess. Algal Res. 2017, 24, 354–359. [Google Scholar] [CrossRef]
- Dresler, S.; Maksymiec, W. Capillary zone electrophoresis for determination of reduced and oxidised ascorbate and glutathione in roots and leaf segments of Zea mays plants exposed to Cd and Cu. Acta Sci. Pol. Hortorum Cultus 2013, 12, 143–155. [Google Scholar]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Cadmium hyperaccumulation leads to an increase of glutathione rather than phytochelatins in the cadmium hyperaccumulator Sedum alfredii. J. Plant. Physiol. 2007, 164, 1489–1498. [Google Scholar] [CrossRef]
- Kutrowska, A.; Szelag, M. Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol. Plant. 2014, 36, 1957–1968. [Google Scholar] [CrossRef] [Green Version]
- Hawrylak-Nowak, B.; Dresler, S.; Matraszek, R. Exogenous malic and acetic acids reduce cadmium phytotoxicity and enhance cadmium accumulation in roots of sunflower plants. Plant. Physiol. Biochem. 2015, 94, 225–234. [Google Scholar] [CrossRef]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Stanisławski, G.; Bany, I.; Wójcik, M. Effect of short-term Zn/Pb or long-term multi-metal stress on physiological and morphological parameters of metallicolous and nonmetallicolous Echium vulgare L. populations. Plant. Physiol. Biochem. 2017, 115, 380–389. [Google Scholar] [CrossRef]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Yu, H.; Li, T.; Zhang, X. Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 150, 168–175. [Google Scholar] [CrossRef]
- Do Nascimento, P.G.G.; Lemos, T.L.G.; Bizerra, A.M.C.; Arriaga, Â.M.C.; Ferreira, D.A.; Santiago, G.M.P.; Braz-Filho, R.; Costa, J.G.M. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, Q.; Wang, Q.; Zhou, L.; Zhang, Z.; Zhang, L.; Huang, T. Effects of lead, copper and cadmium stresses on growth and inherent quality of Prunalla vulgaris. Zhongguo Zhongyao Zazhi 2010, 35, 263–267. [Google Scholar] [PubMed]
- Wang, Q.J.; Lei, X.Y.; Zheng, L.P.; Wang, J.W. Molecular characterization of an elicitor-responsive 3-hydroxy-3-methylglutaryl coenzyme A reductase gene involved in oleanolic acid production in cell cultures of Achyranthes bidentata. Plant. Growth Regul. 2017, 81, 335–343. [Google Scholar] [CrossRef]
- Gupta, P.; Khatoon, S.; Tandon, P.K.; Rai, V. Effect of cadmium on growth, bacoside A, and bacopaside I of Bacopa monnieri (L.), a Memory Enhancing Herb. Sci. World J. 2014, 2014, 824586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, O.T.; Kim, M.Y.; Hong, M.H.; Ahn, J.C.; Hwang, B. Stimulation of asiaticoside accumulation in the whole plant cultures of Centella asiatica (L.) Urban by elicitors. Plant. Cell Rep. 2004, 23, 339–344. [Google Scholar] [CrossRef]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Kocjan, R.; Polkowski, J.; Matkowski, A.; Latalski, M.; et al. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]







| Ca mg·g−1 DW | Mg mg·g−1 DW | |||||
| leaf | root | trichomes | leaf | root | trichomes | |
| Control | 13.1 ± 0.3 b | 5.81 ± 0.37 a | 11.7 ± 0.9 a | 5.53 ± 0.15 a | 4.31 ± 0.92 a | 2.78 ± 0.15 a |
| ChS | 14.6 ± 0.3 a | 4.16 ± 0.16 b | 13.6 ± 0.5 a | 5.58 ± 0.35 a | 2.44 ± 0.21 b | 3.07 ± 0.23 a |
| AS | 13.7 ± 0.4 b | 4.12 ± 0.48 b | 13.7 ± 0.7 a | 4.96 ± 0.22 a | 2.41 ± 0.11 b | 3.04 ± 0.09 a |
| K mg·g−1 DW | Fe µg·g−1 DW | |||||
| leaf | root | trichomes | leaf | root | trichomes | |
| Control | 85.0 ± 3.0 a | 46.5 ± 3.2 a | 28.6 ± 2.0 a | 79.9 ± 3.3 a | 3153 ± 682 a | 129.8 ± 6.3 a |
| ChS | 74.8 ± 4.4 b | 42.4 ± 1.8 a | 29.7 ± 3.7 a | 72.8 ± 6.7 a | 3535 ± 517 a | 116.8 ± 17.6 a |
| AS | 70.9 ± 1.9 b | 45.6 ± 3.9 a | 28.2 ± 1.2 a | 76.8 ± 3.4 a | 3463 ± 325 a | 128.5 ± 6.4 a |
| Cu µg·g−1 DW | Mn µg·g−1 DW | |||||
| leaf | root | trichomes | leaf | root | trichomes | |
| Control | 6.13 ± 0.34 a | 12.1 ± 1.3 a | 6.71 ± 0.85 a | 7.02 ± 0.72 b | 5.80 ± 0.86 b | 7.06 ± 1.40 a |
| ChS | 4.82 ± 0.37 b | 10.6 ± 1.9 a | 4.91 ± 0.47 a | 9.92 ± 1.15 a | 10.10 ± 0.92 a | 7.65 ± 1.42 a |
| AS | 4.56 ± 0.53 b | 11.8 ± 1.3 a | 5.35 ± 0.49 a | 7.35 ± 1.03 b | 9.13 ± 1.32 a | 6.96 ± 0.68 a |
| Zn µg·g−1 DW | Mo µg·g−1 DW | |||||
| leaf | root | trichomes | leaf | root | trichomes | |
| Control | 22.4 ± 2.2 a | 64.0 ± 2.9 a | 25.2 ± 2.8 a | 4.45 ± 0.91 a | 11.51 ± 4.00 a | 2.60 ± 0.48 a |
| ChS | 20.1 ± 0.8 a | 28.1 ± 1.7 b | 17.6 ± 1.9 b | 3.09 ± 0.52 a | 8.79 ± 4.03 a | 2.58 ± 0.39 a |
| AS | 21.5 ± 1.8 a | 53.0 ± 6.6 a | 22.0 ± 3.1 ab | 2.94 ± 0.38 a | 11.26 ± 1.79 a | 2.39 ± 0.21 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresler, S.; Strzemski, M.; Kováčik, J.; Sawicki, J.; Staniak, M.; Wójciak, M.; Sowa, I.; Hawrylak-Nowak, B. Tolerance of Facultative Metallophyte Carlina acaulis to Cadmium Relies on Chelating and Antioxidative Metabolites. Int. J. Mol. Sci. 2020, 21, 2828. https://doi.org/10.3390/ijms21082828
Dresler S, Strzemski M, Kováčik J, Sawicki J, Staniak M, Wójciak M, Sowa I, Hawrylak-Nowak B. Tolerance of Facultative Metallophyte Carlina acaulis to Cadmium Relies on Chelating and Antioxidative Metabolites. International Journal of Molecular Sciences. 2020; 21(8):2828. https://doi.org/10.3390/ijms21082828
Chicago/Turabian StyleDresler, Sławomir, Maciej Strzemski, Jozef Kováčik, Jan Sawicki, Michał Staniak, Magdalena Wójciak, Ireneusz Sowa, and Barbara Hawrylak-Nowak. 2020. "Tolerance of Facultative Metallophyte Carlina acaulis to Cadmium Relies on Chelating and Antioxidative Metabolites" International Journal of Molecular Sciences 21, no. 8: 2828. https://doi.org/10.3390/ijms21082828
APA StyleDresler, S., Strzemski, M., Kováčik, J., Sawicki, J., Staniak, M., Wójciak, M., Sowa, I., & Hawrylak-Nowak, B. (2020). Tolerance of Facultative Metallophyte Carlina acaulis to Cadmium Relies on Chelating and Antioxidative Metabolites. International Journal of Molecular Sciences, 21(8), 2828. https://doi.org/10.3390/ijms21082828