Rapid One-Pot Synthesis of Polydopamine Encapsulated Carbon Anchored with Au Nanoparticles: Versatile Electrocatalysts for Chloramphenicol and Folic Acid Sensors
Abstract
:1. Introduction
2. Results and Discussion
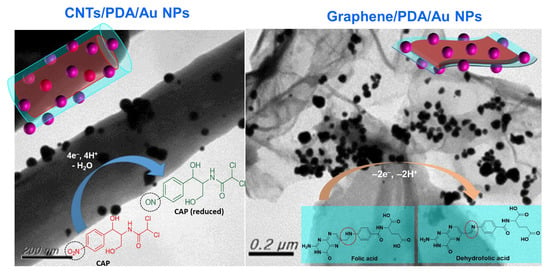
2.1. The Morphology, Structure, and Composition of CNTs/PDA/AuNPs
2.2. Characterizations of rGO/PDA/AuNPs
2.3. Electrocatalytic Sensing Ability of the CNTs/PDA/AuNPs-Modified Electrode to Chloramphenicol
2.4. rGO/PDA/AuNPs Modified Electrode: An Excellent Electrocatalyst for Sensing Folic Acid
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Synthesis of CNTs/PDA/AuNPs
3.3. Synthesis of rGO/PDA/AuNPs
3.4. Fabrication of CNTs/PDA/AuNPs and rGO/PDA/AuNPs Modified Glassy Carbon Electrodes (GCEs)
3.5. Electrochemical Experiments
3.6. Preparation of Real Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, W.; Ratinac, K.R.; Ringer, S.P.; Thordarson, P.; Gooding, J.J.; Braet, F. Carbon nanomaterials in biosensors: Should you use nanotubes or graphene? Angew. Chem. Int. Ed. 2010, 49, 2114–2138. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Y.; Zhu, C.; Li, H.; Du, D.; Lin, Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016, 76, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hou, L.; Samori, P. Coupling carbon nanomaterials with photochromic molecules for the generation of optically responsive materials. Nat. Commun. 2016, 7, 11118. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Govindasamy, M.; Chen, S.-M.; Karthik, R.; Huang, S.-T. Determination of dopamine using a glassy carbon electrode modified with a graphene and carbon nanotube hybrid decorated with molybdenum disulfide flowers. Microchim. Acta 2016, 183, 2267–2275. [Google Scholar] [CrossRef]
- Yan, Q.-L.; Gozin, M.; Zhao, F.-Q.; Cohen, A.; Pang, S.-P. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef] [Green Version]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.-M.; Chen, T.-W.; Kumar, A.S.; Huang, S.-T. Core-shell heterostructured multiwalled carbon nanotubes@reduced graphene oxide nanoribbons/chitosan, a robust nanobiocomposite for enzymatic biosensing of hydrogen peroxide and nitrite. Sci. Rep. 2017, 7, 11910. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zheng, X.; Li, H.-F.; Lin, J.-M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef]
- Komane, P.P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Functionalized, vertically super-aligned multiwalled carbon nanotubes for potential biomedical applications. Int. J. Mol. Sci. 2020, 21, 2276. [Google Scholar] [CrossRef] [Green Version]
- Surya, S.G.; Majhi, S.M.; Agarwal, D.K.; Lahcen, A.A.; Yuvaraja, S.; Chappanda, K.N.; Salama, K.N. A label-free aptasensor FET based on Au nanoparticle decorated Co3O4 nanorods and a SWCNT layer for detection of cardiac troponin T protein. J. Mat. Chem. B 2020, 8, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef]
- Ai, L.; Tian, T.; Jiang, J. Ultrathin graphene layers encapsulating nickel nanoparticles derived metal–organic frameworks for highly efficient electrocatalytic hydrogen and oxygen evolution reactions. ACS Sustain. Chem. Eng. 2017, 5, 4771–4777. [Google Scholar] [CrossRef]
- Mani, V.; Chen, S.-M.; Lou, B.-S. Three dimensional graphene oxide-carbon nanotubes and graphene-carbon nanotubes hybrids. Int. J. Electrochem. Sci. 2013, 8, 60. [Google Scholar]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xiao, C.; Chen, X.; Gao, R.; Ding, S. Porous γ-Fe2O3 spheres coated with N-doped carbon from polydopamine as Li-ion battery anode materials. Nanotechnology 2016, 27, 215403. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, S.; Liu, X. Efficient one-pot synthesis of mussel-inspired molecularly imprinted polymer coated graphene for protein-specific recognition and fast separation. J. Phy. Chem. C 2013, 117, 18448–18456. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.-M. Highly selective amperometric sensor for the trace level detection of hydrazine at bismuth nanoparticles decorated graphene nanosheets modified electrode. Talanta 2014, 124, 43–51. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Mani, V.; Chen, S.-M.; Huang, W.-T.; Jen, J.-F. Rapid microwave assisted synthesis of graphene nanosheets/polyethyleneimine/gold nanoparticle composite and its application to the selective electrochemical determination of dopamine. Talanta 2014, 120, 148–157. [Google Scholar] [CrossRef]
- Rao, C.R.; Kulkarni, G.U.; Thomas, P.J.; Edwards, P.P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Min, D.-H. Preparation of the hybrid film of poly (allylamine hydrochloride)-functionalized graphene oxide and gold nanoparticle and its application for laser-induced desorption/ionization of small molecules. Langmuir 2012, 28, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Y.; Qu, J.; Qu, J.; Li, S. Selective sensing of hydroquinone and catechol based on multiwalled carbon nanotubes/polydopamine/gold nanoparticles composites. Sens. Actuators B 2016, 223, 501–508. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Wang, H.-B.; Gan, T.; Wu, Y.-Y.; Li, J.; Liu, Y.-M. Electrochemical biosensor based on silver nanoparticles–polydopamine–graphene nanocomposite for sensitive determination of adenine and guanine. Talanta 2013, 114, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Liu, Y.-J.; Wang, H.-B.; Wang, Y.-Y. A sensitive electrochemical DNA biosensor based on silver nanoparticles-polydopamine@ graphene composite. Electrochim. Acta 2014, 118, 130–137. [Google Scholar] [CrossRef]
- Liu, Y.; Han, Y.; Chen, R.; Zhang, H.; Liu, S.; Liang, F. In situ immobilization of copper nanoparticles on polydopamine coated graphene oxide for H2O2 determination. PLoS ONE 2016, 11, e0157926. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A double signal electrochemical human immunoglobulin G immunosensor based on gold nanoparticles-polydopamine functionalized reduced graphene oxide as a sensor platform and AgNPs/carbon nanocomposite as signal probe and catalytic substrate. Biosens. Bioelectron. 2016, 77, 1078–1085. [Google Scholar] [CrossRef]
- Rajaji, U.; Manavalan, S.; Chen, S.-M.; Govindasamy, M.; Chen, T.-W.; Maiyalagan, T. Microwave-assisted synthesis of europium(III) oxide decorated reduced graphene oxide nanocomposite for detection of chloramphenicol in food samples. Compos. Part B 2019, 161, 29–36. [Google Scholar] [CrossRef]
- Wang, M.-H.; Gu, J.-A.; Mani, V.; Wu, Y.-C.; Lin, Y.-J.; Chia, Y.-M.; Huang, S.-T. A rapid fluorescence detecting platform: Applicable to sense carnitine and chloramphenicol in food samples. RSC Adv. 2014, 4, 64112–64118. [Google Scholar] [CrossRef]
- Alibolandi, M.; Hadizadeh, F.; Vajhedin, F.; Abnous, K.; Ramezani, M. Design and fabrication of an aptasensor for chloramphenicol based on energy transfer of CdTe quantum dots to graphene oxide sheet. Mater. Sci. Eng. C 2015, 48, 611–619. [Google Scholar] [CrossRef]
- Borowiec, J.; Wang, R.; Zhu, L.; Zhang, J. Synthesis of nitrogen-doped graphene nanosheets decorated with gold nanoparticles as an improved sensor for electrochemical determination of chloramphenicol. Electrochim. Acta 2013, 99, 138–144. [Google Scholar] [CrossRef]
- Rajaji, U.; Muthumariappan, A.; Chen, S.-M.; Chen, T.-W.; Tseng, T.-W.; Wang, K.; Qi, D.; Jiang, J. Facile sonochemical synthesis of porous and hierarchical manganese(III) oxide tiny nanostructures for super sensitive electrocatalytic detection of antibiotic (chloramphenicol) in fresh milk. Ultrason. Sonochem. 2019, 58, 104648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Anwar, A.; Kanwal, Q. Electrochemical determination of folic acid: A short review. Anal. Biochem. 2016, 510, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, S.; Mani, V.; Jeromiyas, N.; Ponnusamy, V.K.; Yoshimura, M. A composite film prepared from titanium carbide Ti3C2Tx (MXene) and gold nanoparticles for voltammetric determination of uric acid and folic acid. Microchim. Acta 2020, 187, 33. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, P.; John, S.A. Selective electrochemical sensor for folic acid at physiological pH using ultrathin electropolymerized film of functionalized thiadiazole modified glassy carbon electrode. Biosens. Bioelectron. 2009, 24, 3575–3580. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, M.P.; Desai, P.B.; Srivastava, A.K. Simultaneous electro-catalytic oxidative determination of ascorbic acid and folic acid using Fe3O4 nanoparticles modified carbon paste electrode. J. Electroanal. Chem. 2015, 741, 71–79. [Google Scholar] [CrossRef]
- Mani, V.; Umamaheswari, R.; Chen, S.-M.; Govindasamy, M.; Su, C.; Sathiyan, A.; Merlin, J.P.; Keerthi, M. Highly sensitive determination of folic acid using graphene oxide nanoribbon film modified screen printed carbon electrode. Int. J. Electrochem. Sci. 2017, 12, 475–484. [Google Scholar] [CrossRef]
- Govindasamy, M.; Manavalan, S.; Chen, S.M.; Rajaji, U.; Chen, T.W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. Determination of neurotransmitter in biological and drug samples using gold nanorods decorated f-MWCNTs modified electrode. J. Electrochem. Soc. 2018, 165, B370–B377. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Mani, V.; Chen, S.-M.; Viswanath, B.; Vasantha, V.S.; Govindasamy, M. Electrodeposition of gold nanoparticles on a pectin scaffold and its electrocatalytic application in the selective determination of dopamine. RSC Adv. 2014, 4, 55900–55907. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.-M.; Subramani, B.; Sathiyan, A.; Merlin, J.P. Determination of folic acid using graphene/molybdenum disulfide nanosheets/gold nanoparticles ternary composite. Int. J. Electrochem. Sci. 2017, 12, e267. [Google Scholar] [CrossRef]
- Govindasamy, M.; Chen, S.-M.; Mani, V.; Devasenathipathy, R.; Umamaheswari, R.; Santhanaraj, K.J.; Sathiyan, A. Molybdenum disulfide nanosheets coated multiwalled carbon nanotubes composite for highly sensitive determination of chloramphenicol in food samples milk, honey and powdered milk. J. Colloid Interface Sci. 2017, 485, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.-C.; Zhang, J.-W. A highly selective electrochemical sensor for chloramphenicol based on three-dimensional reduced graphene oxide architectures. Talanta 2016, 161, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhao, J.; Chen, M.; Yang, T.; Luo, S.; Jiao, K. Electrocatalytic determination of chloramphenicol based on molybdenum disulfide nanosheets and self-doped polyaniline. Talanta 2015, 131, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Jakubec, P.; Urbanová, V.; Medříková, Z.; Zbořil, R. Advanced sensing of antibiotics with magnetic gold nanocomposite: Electrochemical detection of chloramphenicol. Chem. Eur. J. 2016, 22, 14279–14284. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Govindasamy, M.; Chen, S.-M.; Mani, V.; Lou, B.-S.; Devasenathipathy, R.; Hou, Y.-S.; Elangovan, A. Green synthesized gold nanoparticles decorated graphene oxide for sensitive determination of chloramphenicol in milk, powdered milk, honey and eye drops. J. Colloid Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef]
- Agüı, L.; Guzmán, A.; Yáñez-Sedeño, P.; Pingarrón, J. Voltammetric determination of chloramphenicol in milk at electrochemically activated carbon fibre microelectrodes. Anal. Chim. Acta 2002, 461, 65–73. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Chen, T.-T.; Wang, J.-Y.; Fang, H.-L.; Fan, D.-H.; Wang, W. UV-assisted synthesis of tetrapods-like titanium nitride-reduced graphene oxide nanohybrids for electrochemical determination of chloramphenicol. Sens. Actuators B 2016, 225, 298–304. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Hatami, M.; Moradi, R.; Khalilzadeh, M.A.; Amiri, S.; Sadeghifar, H. Synergic effect of Pt-Co nanoparticles and a dopamine derivative in a nanostructured electrochemical sensor for simultaneous determination of N-acetylcysteine, paracetamol and folic acid. Microchim. Acta 2016, 183, 2957–2964. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, X.; Ma, W.; Li, L.; Zhang, Y. Voltammetric determination of folic acid using adsorption of methylene blue onto electrodeposited of reduced graphene oxide film modified glassy carbon electrode. Electroanalysis 2016, 28, 312–319. [Google Scholar] [CrossRef]
- Cinková, K.; Švorc, Ľ.; Šatkovská, P.; Vojs, M.; Michniak, P.; Marton, M. Simple and rapid quantification of folic acid in pharmaceutical tablets using a cathodically pretreated highly boron-doped polycrystalline diamond electrode. Anal. Lett. 2016, 49, 107–121. [Google Scholar] [CrossRef]
- Chekin, F.; Teodorescu, F.; Coffinier, Y.; Pan, G.-H.; Barras, A.; Boukherroub, R.; Szunerits, S. MoS2/reduced graphene oxide as active hybrid material for the electrochemical detection of folic acid in human serum. Biosens. Bioelectron. 2016, 85, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Karimi-Maleh, H. Modified multiwall carbon nanotubes paste electrode as a sensor for simultaneous determination of 6-thioguanine and folic acid using ferrocenedicarboxylic acid as a mediator. J. Electroanal. Chem. 2010, 640, 75–83. [Google Scholar] [CrossRef]
- Abdelwahab, A.A.; Shim, Y.-B. Simultaneous determination of ascorbic acid, dopamine, uric acid and folic acid based on activated graphene/MWCNT nanocomposite loaded Au nanoclusters. Sens. Actuators B 2015, 221, 659–665. [Google Scholar] [CrossRef]







| Electrode | Linear Range/µM | LOD/nM | Methods | Ref. |
|---|---|---|---|---|
| 3D reduced GO | 1–113 | 150 | Differential pulse voltammetry | [42] |
| MoS2/self-doped polyaniline | 0.1–1000 | 65 | Differential pulse voltammetry | [43] |
| Fe3O4-carboxymethyl cellulose/Au | 2.5–25 | 66 | Square wave voltammetry | [44] |
| AuNPs/GO | 1.5–2.95 | 250 | Amperometry | [45] |
| Activated carbon fiber microelectrodes | 0.1–10 | 47 | Square wave voltammetry | [46] |
| Titanium nitride-rGO | 0.05–100 | 20 | Voltammetry | [47] |
| N-doped graphene/AuNPs | 2–80 | 59 | Linear sweep voltammetry | [30] |
| CNTs/PDA/AuNPs | 0.1–534 | 36 | Amperometry | This work |
| Samples | Added/µM | Found/µM | Recovery/% | RSD */% |
|---|---|---|---|---|
| Milk | 1 | 0.96 | 96.0 | 2.88 |
| 5 | 4.92 | 98.4 | 2.93 | |
| 10 | 9.63 | 96.3 | 3.50 | |
| Powdered milk | 1 | 0.97 | 97.0 | 3.92 |
| 5 | 4.83 | 96.6 | 3.52 | |
| 10 | 9.73 | 97.3 | 2.74 | |
| Honey | 1 | 0.95 | 95.0 | 4.11 |
| 5 | 4.80 | 96.0 | 3.4 | |
| 10 | 9.7 | 97.0 | 3.63 |
| Electrode | Linear Range/µM | LOD/µM | Ref. |
|---|---|---|---|
| Carbon paste electrode/Pt-Co nanoparticles/2-(3,4-dihydroxyphenethyl)isoindoline-1,3-dione | 2–550 | 0.8 | [48] |
| Methylene blue/rGO | 4–167 | 0.5 | [49] |
| Fe3O4 nanoparticles | 0.065–98 | 0.002 | [36] |
| B-doped polycrystalline diamond | 0.1–167 | 0.03 | [50] |
| MoS2/rGO | 0.01–100 | 0.01 | [51] |
| ferrocenedicarboxylic acid–MWCNTs | 4.6–152 | 1.1 | [52] |
| Au nanoclusters-activated graphene/MWCNT | 10–170 | 0.09 | [53] |
| rGO/PDA/AuNPs | 0.1–905 | 0.025 | This work |
| Samples | Added/µM | Found/µM | Recovery/% | RSD */% |
|---|---|---|---|---|
| Human serum | 5 | 4.76 | 95.2 | 2.58 |
| 10 | 9.77 | 97.7 | 3.53 | |
| Human urine | 5 | 4.85 | 97.0 | 2.63 |
| 10 | 9.73 | 97.3 | 3.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, V.; Balamurugan, T.S.T.; Huang, S.-T. Rapid One-Pot Synthesis of Polydopamine Encapsulated Carbon Anchored with Au Nanoparticles: Versatile Electrocatalysts for Chloramphenicol and Folic Acid Sensors. Int. J. Mol. Sci. 2020, 21, 2853. https://doi.org/10.3390/ijms21082853
Mani V, Balamurugan TST, Huang S-T. Rapid One-Pot Synthesis of Polydopamine Encapsulated Carbon Anchored with Au Nanoparticles: Versatile Electrocatalysts for Chloramphenicol and Folic Acid Sensors. International Journal of Molecular Sciences. 2020; 21(8):2853. https://doi.org/10.3390/ijms21082853
Chicago/Turabian StyleMani, Veerappan, T.S.T. Balamurugan, and Sheng-Tung Huang. 2020. "Rapid One-Pot Synthesis of Polydopamine Encapsulated Carbon Anchored with Au Nanoparticles: Versatile Electrocatalysts for Chloramphenicol and Folic Acid Sensors" International Journal of Molecular Sciences 21, no. 8: 2853. https://doi.org/10.3390/ijms21082853
APA StyleMani, V., Balamurugan, T. S. T., & Huang, S. -T. (2020). Rapid One-Pot Synthesis of Polydopamine Encapsulated Carbon Anchored with Au Nanoparticles: Versatile Electrocatalysts for Chloramphenicol and Folic Acid Sensors. International Journal of Molecular Sciences, 21(8), 2853. https://doi.org/10.3390/ijms21082853