Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma
Abstract
:1. Introduction
2. Results
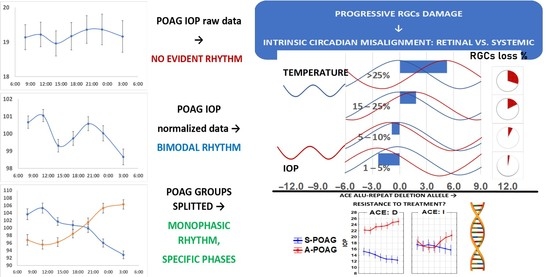
2.1. IOP Variability and Circadian Rhythm: Raw vs. Normalized Data Analysis
2.2. IOP Circadian Phase in POAG Progression and Its Relationship to Marker Circadian Rhythm (Body Temperature, Tb) and Sleep
2.3. Candidate Gene Polymorphisms Involved in IOP 24-h Variability Modulation
3. Discussion
4. Materials and Methods
4.1. RGC Function and Damage Assessment
4.2. POAG Diagnosis and Progression Criteria
4.3. Intraocular Pressure Measurements
4.4. Body Tempeature (Tb) Measurements
4.5. Sleep Assessment
4.6. Chronotype Assessment
4.7. Genotyping
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ψ | intrinsic phase angle between intraocular pressure and body temperature circadian rhythms |
| A-POAG | Advanced Primary Open-Angle Glaucoma |
| ACE | Angiotensin Converting Enzyme |
| ASP | Average Sleep Phase (of the three-day self-reported sleep assessment) |
| BMI | Body Mass Index |
| FLV | Focal Loss Volume |
| GLV | Global Loss Volume |
| IOP | Intraocular pressure |
| MEQ | Morningness-Eveningness Questionnaire |
| MESOR | Midline Estimating Statistics of Rhythm, or 24-h cosine adjusted mean |
| OD | right eye (Oculus Dexter) |
| ONH | Optic Nerve Head |
| OS | left eye (Oculus Sinister) |
| NPDS | Non-Penetrating Deep Sclerectomy |
| PERG | Pattern Electroretinogram |
| POAG | Primary Open-Angle Glaucoma |
| RNFL | Retinal Nerve Fiber Layer |
| RGCs | Retinal Ganglion Cells |
| SAP mD | Standard Automated Perimetry mean Deviation |
| SCN | Suprachiasmatic nucleus |
| S-POAG | Stable Primary Open-Angle Glaucoma |
| STE | Sinustrabeculectomy |
| Tb phi | Body temperature circadian phase |
References
- Aptel, F.; Weinreb, R.N.; Chiquet, C.; Mansouri, K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog. Retin. Eye Res. 2016, 55, 108–148. [Google Scholar] [CrossRef]
- Konstas, A.G.; Kahook, M.Y.; Araie, M.; Katsanos, A.; Quaranta, L.; Rossetti, L.; Holló, G.; Detorakis, E.T.; Oddone, F.; Mikropoulos, D.G.; et al. Diurnal and 24-h Intraocular Pressures in Glaucoma: Monitoring Strategies and Impact on Prognosis and Treatment. Adv. Ther. 2018, 35, 1775–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.W.; Park, K.H. Exogenous influences on intraocular pressure. Br. J. Ophthalmol. 2019, 103, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.H.; Kripke, D.F.; Hoffman, R.E.; Twa, M.D.; Loving, R.T.; Rex, K.M.; Gupta, N.; Weinreb, R.N. Nocturnal elevation of intraocular pressure in young adults. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2707–2712. [Google Scholar]
- Cvenkel, B.; Atanasovska Velkovska, M. Self-monitoring of intraocular pressure using Icare HOME tonometry in clinical practice. Clin. Ophthalmol. 2019, 13, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Fogagnolo, P.; Orzalesi, N.; Ferreras, A.; Rossetti, L. The circadian curve of intraocular pressure: Can we estimate its characteristics during office hours? Investig. Ophthalmol. Vis. Sci. 2009, 50, 2209–2215. [Google Scholar] [CrossRef]
- Mansouri, K.; Weinreb, R.N.; Liu, J.H. Effects of aging on 24-hour intraocular pressure measurements in sitting and supine body positions. Investig. Ophthalmol. Vis. Sci. 2012, 53, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Baig, N.; Hansapinyo, L.; Jhanji, V.; Wei, S.; Tham, C.C. Comparison of self-measured diurnal intraocular pressure profiles using rebound tonometry between primary angle closure glaucoma and primary open angle glaucoma patients. PLoS ONE 2017, 12, e0173905. [Google Scholar] [CrossRef]
- Perlman, J.I.; Delany, C.M.; Sothern, R.B.; Skolnick, K.A.; Murray, D.; Jacobs, R.W.; Shue, J.L.; Kaplan, E.; Friedman, N.C.; Nemchausky, B.A.; et al. Relationships between 24 h observations in intraocular pressure vs blood pressure, heart rate, nitric oxide and age in the medical chronobiology aging project. Clin. Ter. 2007, 158, 31–47. [Google Scholar]
- Jonas, J.B.; Budde, W.M.; Stroux, A.; Oberacher-Velten, I.M.; Juenemann, A.G. Circadian intraocular pressure profiles in chronic open angle glaucomas. J. Ophthalmic Vis. Res. 2010, 5, 92–100. [Google Scholar]
- Huang, J.; Katalinic, P.; Kalloniatis, M.; Hennessy, M.P.; Zangerl, B. Diurnal Intraocular Pressure Fluctuations with Self-tonometry in Glaucoma Patients and Suspects: A Clinical Trial. Optom. Vis. Sci. 2018, 95, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottet, B.; Chiquet, C.; Aptel, F.; Noel, C.; Gronfier, C.; Buguet, A.; Romanet, J.P. 24-hour intraocular pressure of young healthy humans in supine position: Rhythm and reproducibility. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8186–8191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.H.K.; Bouligny, R.P.; Kripke, D.F.; Weinreb, R.N. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4439–4442. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Liu, J.H.K.; Weinreb, R.N.; Tafreshi, A.; Medeiros, F.A. Analysis of continuous 24-hour intraocular pressure patterns in glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 8050–8056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnifili, L.; Mastropasqua, R.; Frezzotti, P.; Fasanella, V.; Motolese, I.; Pedrotti, E.; Di Iorio, A.; Mattei, P.A.; Motolese, E.; Mastropasqua, L. Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol. 2015, 93, e14–e21. [Google Scholar] [CrossRef]
- Aptel, F.; Musson, C.; Zhou, T.; Lesoin, A.; Chiquet, C. 24-Hour intraocular pressure rhythm in patients with untreated primary open angle glaucoma and effects of selective laser trabeculoplasty. J. Glaucoma 2017, 26, 272–277. [Google Scholar] [CrossRef]
- Posarelli, C.; Ortenzio, P.; Ferreras, A.; Toro, M.D.; Passani, A.; Loiudice, P.; Oddone, F.; Casini, G.; Figus, M. Twenty-Four-Hour Contact Lens Sensor Monitoring of Aqueous Humor Dynamics in Surgically or Medically Treated Glaucoma Patients. J. Ophthalmol. 2019, 2019, 9890831. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Mansouri, K.; Weinreb, R.N. Estimation of 24-Hour Intraocular Pressure Peak Timing and Variation Using a Contact Lens Sensor. PLoS ONE 2015, 10, e0129529. [Google Scholar] [CrossRef] [Green Version]
- Buys, Y.M.; Alasbali, T.; Jin, Y.P.; Smith, M.; Gouws, P.; Geffen, N.; Flanagan, J.G.; Shapiro, C.M.; Trope, G.E. Effect of sleeping in a head-up position on intraocular pressure in patients with glaucoma. Ophthalmology 2010, 117, 1348–1351. [Google Scholar] [CrossRef]
- Xu, S.; Jiao, Q.; Cheng, Y.; Sun, J.; Lu, Q.; Zhong, Y. Short-Term Reproducibility of Twenty-Four-Hour Intraocular Pressure Curves in Untreated Patients with Primary Open-Angle Glaucoma and Ocular Hypertension. PLoS ONE 2015, 10, e0140206. [Google Scholar] [CrossRef]
- Gao, Y.; Wan, B.; Li, P.; Zhang, Y.; Tang, X. Short-term reproducibility of intraocular pressure and ocular perfusion pressure measurements in Chinese volunteers and glaucoma patients. BMC Ophthalmol. 2016, 16, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubin, D.G.; Weinert, D.; Bolotnova, T.N. Age-dependent Changes of the Temporal Order—Causes and Treatment. Curr. Aging Sci. 2016, 9, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Drouyer, E.; Dkhissi-Benyahya, O.; Chiquet, C.; WoldeMussie, E.; Ruiz, G.; Wheeler, L.A.; Denis, P.; Cooper, H.M. Glaucoma alters the circadian timing system. PLoS ONE 2008, 3, e3931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardin, J.-L.; Zizi, F.; Lazzaro, D.R.; Wolintz, A.H. Circadian rhythm dysfunction in glaucoma: A hypothesis. J. Circadian Rhythm. 2008, 6, 1. [Google Scholar]
- Gubin, D.G.; Malishevskaya, T.N.; Astakhov, Y.S.; Astakhov, S.Y.; Cornelissen, G.; Kuznetsov, V.A.; Weinert, D. Progressive retinal ganglion cell loss in primary open-angle glaucoma is associated with temperature circadian rhythm phase delay and compromised sleep. Chronobiol. Int. 2019, 36, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Feigl, B.; Mattes, D.; Thomas, R.; Zele, A.J. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4362–4367. [Google Scholar] [CrossRef] [Green Version]
- Gracitelli, C.P.; Duque-Chica, G.L.; Roizenblatt, M.; Moura, A.L.; Nagy, B.V.; Ragot de Melo, G.; Borba, P.D.; Teixeira, S.H.; Tufik, S.; Ventura, D.F. Intrinsically photosensitive retinal ganglion cell activity is associated with decreased sleep quality in patients with glaucoma. Ophthalmology 2015, 122, 1139–1148. [Google Scholar] [CrossRef]
- Obara, E.A.; Hannibal, J.; Heegaard, S.; Fahrenkrug, J. Loss of melanopsin-expressing retinal ganglion cells in severely staged glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4661–4667. [Google Scholar] [CrossRef] [Green Version]
- Lax, P.; Ortuño-Lizarán, I.; Maneu, V.; Vidal-Sanz, M.; Cuenca, N. Photosensitive Melanopsin-Containing Retinal Ganglion Cells in Health and Disease: Implications for Circadian Rhythms. Int. J. Mol. Sci. 2019, 20, 3164. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Provencio, I.; Tu, D.C.; Pires, S.S.; Rollag, M.D.; Castrucci, A.M.; Pletcher, M.T.; Sato, T.K.; Wiltshire, T.; Andahazy, M.; et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003, 301, 525–527. [Google Scholar] [CrossRef] [Green Version]
- Bertolucci, C.; Giudice, E.; Fazio, F.; Piccione, G. Circadian intraocular pressure rhythms in athletic horses under different lighting regime. Chronobiol. Int. 2009, 26, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Giannetto, C.; Fazio, F.; Giudice, E. Influence of different artificial lighting regimes on intraocular pressure circadian profile in the dog (Canis familiaris). Exp. Anim. 2010, 59, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braslow, R.A.; Gregory, D.S. Adrenergic decentralization modifies the circadian rhythm of intraocular pressure. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1730–1732. [Google Scholar]
- Lozano, D.C.; Hartwick, A.T.E.; Twa, M.D. Circadian rhythm of intraocular pressure in the adult rat. Chronobiol. Int. 2015, 32, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besharse, J.C.; McMahon, D.G. The Retina and other Light Sensitive Ocular Clocks. J. Biol. Rhythm. 2016, 31, 223–243. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Buhr, E.D.; Higashide, T.; Sugiyama, K.; Van Gelder, R.N. Light entrainment of the murine intraocular pressure circadian rhythm utilizes non-local mechanisms. PLoS ONE 2017, 12, e0184790. [Google Scholar] [CrossRef]
- Gubin, D.; Cornelissen, G.; Weinert, D.; Vetoshkin, A.S.; Gapon, L.I.; Shurkevich, N.P.; Poshinov, F.A.; Belozerova, N.V.; Danilova, L.A. Circadian disruption and Vascular Variability Disorders (VVD)—Mechanisms linking aging, disease state and Arctic shift-work: Applications for chronotherapy. World Heart J. 2013, 5, 285–306. [Google Scholar]
- Liu, J.H.; Weinreb, R.N. Asymmetry of habitual 24-hour intraocular pressure rhythm in glaucoma patients. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7398–7402. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.H.; Sit, A.J.; Weinreb, R.N. Variation of 24-hour intraocular pressure in healthy individuals: Right eye versus left eye. Ophthalmology 2005, 112, 1670–1675. [Google Scholar] [CrossRef]
- Choudhary, R.; Kapoor, M.S.; Singh, A.; Bodakhe, S.H. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthalmol. 2016, 29, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Cho, C.S.; Han, K.; Lee, J. Structural Variation of Alu Element and Human Disease. Genom. Inform. 2016, 14, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, R.; Sekar, D.; Karunanithy, S.; Subramanium, S. Association of angiotensin converting enzyme gene insertion/deletion polymorphism with essential hypertension in south Indian population. Genes Dis. 2016, 3, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Mafra, F.F.P.; Gattai, P.P.; Macedo, M.M.; Mori, M.A.; Araujo, R.C. The angiotensin-I-converting enzyme insertion/deletion in polymorphic element codes for an AluYa5 RNA that downregulates gene expression. Pharm. J. 2018, 18, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Aptel, F.; Tamisier, R.; Pepin, J.-L.; Mottet, B.; Hubanova, R.; Romanet, J.P.; Chiquet, C. Hourly awakening vs continuous contact lens sensor measurements of 24-hour intraocular pressure. Effect on sleep macrostructure and intraocular pressure rhythm. JAMA Ophthalmol. 2014, 132, 1232–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felder-Schmittbuhl, M.P.; Buhr, E.D.; Dkhissi-Benyahya, O.; Hicks, D.; Peirson, S.N.; Ribelayga, C.P.; Sandu, C.; Spessert, R.; Tosini, G. Ocular Clocks: Adapting Mechanisms for Eye Functions and Health. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4856–4870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, S.; Watanabe, M.; Inatomi, S.; Umeda, K.; Yoshida, K.; Ohguro, I.; Ohguro, H. Effects of several anti-glaucoma medications on the circadian intraocular pressure fluctuations in patients with primary open-angle glaucoma. J. Ocul. Pharmacol. Ther. 2014, 30, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Nakamoto, K.; Horiguchi, H.; Ogawa, S.; Noro, T.; Sato, M.; Nakano, T.; Tsuneoka, H.; Yasuda, N. Twenty-Four-Hour Variation of Intraocular Pressure in Primary Open-Angle Glaucoma Treated with Triple Eye Drops. J. Ophthalmol. 2017, 2017, 4398494. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.; Quaranta, L.; Bozkurt, B.; Katsanos, A.; Garcia-Feijoo, J.; Rossetti, L.; Shaarawy, T.; Pfeiffer, N.; Miglior, S. 24-h Efficacy of Glaucoma Treatment Options. Adv. Ther. 2016, 33, 481–517. [Google Scholar] [CrossRef] [Green Version]
- Stoschitzky, K.; Sakotnik, A.; Lercher, P.; Zweiker, R.; Maier, R.; Liebmann, P.; Lindner, W. Influence of beta-blockers on melatonin release. Eur. J. Clin. Pharmacol. 1999, 55, 111–115. [Google Scholar] [CrossRef]
- Ventura, L.M.; Porciatti, V.; Ishida, K.; Feuer, W.J.; Parrish, R.K. Pattern electroretinogram abnormality and glaucoma. Ophthalmology 2005, 112, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Wilsey, L.J.; Fortune, B. Electroretinography in glaucoma diagnosis. Curr. Opin. Ophthalmol. 2016, 27, 118–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmor, M.F.; Zrenner, E. Standard for clinical electroretinography. Doc. Ophthalmol. 1999, 97, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Aptel, F.; Aryal-Charles, N.; Giraud, J.M.; El Chehab, H.; Delbarre, M.; Chiquet, C.; Romanet, J.P.; Renard, J.P. Progression of visual field in patients with primary open-angle glaucoma—ProgF study 1. Acta Ophthalmol. 2015, 93, e615–e620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussel, I.I.; Wollstein, G.; Schuman, J.S. OCT for glaucoma diagnosis, screening and detection of glaucoma progression. Br. J. Ophthalmol. 2014, 98 (Suppl. S2), ii15–ii19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubin, D.G.; Gubin, G.D.; Gapon, L.I.; Weinert, D. Daily melatonin administration attenuates age- dependent disturbances of cardiovascular rhythms. Curr. Aging Sci. 2016, 9, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Gubin, G.D.; Waterhouse, J.; Weinert, D. The circadian body temperature rhythm in the elderly: Effect of single daily melatonin dosing. Chronobiol. Int. 2006, 23, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Gubin, D.G.; Weinert, D.; Rybina, S.V.; Danilova, L.A.; Solovieva, S.V.; Durov, A.M.; Prokopiev, N.Y.; Ushakov, P.A. Activity, sleep and ambient light have a different impact on circadian blood pressure, heart rate and body temperature rhythms. Chronobiol. Int. 2017, 34, 632–649. [Google Scholar] [CrossRef]
- Gubin, D.; Nelaeva, A.; Uzhakova, A.; Hasanova, Y.V.; Cornelissen, G.; Weinert, D. Disrupted circadian rhythms of body temperature, heart rate and fasting blood glucose in prediabetes and type 2 diabetes mellitus. Chronobiol. Int. 2017, 34, 1136–1148. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef] [Green Version]
- Bingham, C.; Arbogast, B.; Cornelissen-Guillaume, G.C.; Lee, J.K.; Halberg, F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 1982, 9, 397–439. [Google Scholar] [PubMed]






| Stable POAG | Advanced POAG | p-Value | |
|---|---|---|---|
| General Characteristics | |||
| Age, years. | 67.61 (60.05; 75.17) | 69.98 (61.83; 78.13) | 0.100 § |
| Number, Sex | n = 65, 46 women | n = 50, 31 women | 0.322 # |
| BMI, kg/m2 | 24.66 (23.91; 25.41) | 24.77 (23.95; 25.60) | 0.852 § |
| Clinical Characteristics | |||
| Global Loss Volume mean, % | 5.95 (5.50; 6.41) | 24.26 (22.82; 25.71) | <0.001 § |
| Global Loss Volume right eye, % | 5.75 (5.32; 6.18) | 20.05 (18.43; 21.67) | <0.001 §,* |
| Global Loss Volume left eye, % | 6.15 (5.60; 6.72) | 28.49 (26.33; 30.64) | <0.001 §,* |
| Focal Loss Volume mean, % | 3.20 (2.72; 3.68) | 10.81 (10.13; 11.48) | <0.001 § |
| Focal Loss Volume right eye, % | 2.95 (2.49; 3.41) | 9.30 (8.75; 9.85) | <0.001 §,* |
| Focal Loss Volume left eye, % | 3.31 (2.79; 3.84) | 12.31 (11.29; 13.34) | <0.001 §,* |
| SAP mD mean, dB | −3.42 (−3.92; −2.91) | −11.95(−13.23;−10.67) | <0.001 § |
| SAP mD right eye, dB | −3.43 (−3.94; −2.92) | −8.04 (−9.71; −6.37) | <0.001 §,* |
| SAP mD left eye, dB | −3.41 (−4.09; −2.72) | −15.86 (−17.68;−14.03) | <0.001 §,* |
| PERG P50A mean, μV | 2.22 (2.02; 2.43) | 1.06 (0.92; 1.21) | <0.001 § |
| PERG P50A right eye, μV | 2.24 (2.03; 2.45) | 1.28 (1.09; 1.47) | <0.001 §,* |
| PERG P50A left eye, μV | 2.21 (2.01; 2.41) | 0.85 (0.68; 1.02) | <0.001 §,* |
| IOP right eye mean, mmHg | 16.29 (15.56; 17.03) | 22.03 (21.00; 23.06) | <0.001 §,* |
| IOP left eye mean, mmHg | 16.10 (15.25; 16.96) | 24.16 (23.16; 25.16) | <0.001 §,* |
| Stable POAG | Advanced POAG | p-Value | |
|---|---|---|---|
| IOP Variability and 24-h Characteristics | |||
| IOPOD SD, % | 11.80 (10.58; 13.03) | 11.44 (10.07; 12.81) | 0.666 § |
| IOPOS SD, % | 11.96 (10.97; 12.96) | 10.73 (9.27; 12.20) | 0.012 § |
| IOPOD 24-h Amplitude, % | 8.52 (7.21; 9.84) | 7.70 (5.93; 9.48) | 0.099 § |
| IOPOS 24-h Amplitude, % | 7.33 (6.11; 8.56) | 7.62 (5.98; 9.26) | 0.778 § |
| IOPOD 24-h phase, hour:min | 12:24 (11:32; 13:36) | 0:12 (23:00; 1:16) | <0.001 $ |
| IOPOS 24-h phase, hour:min | 12:40 (11:24; 14:16) | 0:48 (23:40; 1:36) | <0.001 $ |
| Temperature, Sleep and Phase-Alignment Characteristics | |||
| Bedtime Mean, hour:min | 22:08 (21:44; 22:33) | 23:02 (21:55; 00:10) | <0.001 § |
| Mean Time of Awakening, hour:min | 5:22 (4:20; 6:15) | 5:29 (4:10; 6:47) | 0.984 § |
| Sleep Duration mean, hour:min | 7:13 (6:29; 7:58) | 6:18 (5:22; 7:14) | <0.001 § |
| Sleep Phase mean, hour:min | 1:45 (1:10; 2:19) | 2:15 (1:05; 3:26) | 0.002 § |
| Tb phase, hour:min | 14:54 (14:24; 15:32) | 19:44 (17:32; 21:12) | <0.001 § |
| MEQ, Score | 60.52 (57.12; 63.92) | 66.22 (62.11; 70.32) | 0.033 § |
| IOPOD–ASP Ψ, hour:min | 9:00 (8:13; 9:48) | 4:38 (3:47; 5:32) | <0.001 §,* |
| IOPOS–ASP Ψ, hour:min | 9:08 (8:26; 9:51) | 3:31 (2:46; 4:16) | <0.001 §,* |
| IOPOD–Tb Ψ, hour:min | −1:08 (−2:09; −0:06) | 2:27 (1:02; 3:52) | <0.001 § |
| IOPOS–Tb Ψ, hour:min | −1:21 (−2:18; −0:24) | 3:09 (1:30; 4:32) | <0.001 § |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neroev, V.; Malishevskaya, T.; Weinert, D.; Astakhov, S.; Kolomeichuk, S.; Cornelissen, G.; Kabitskaya, Y.; Boiko, E.; Nemtsova, I.; Gubin, D. Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma. Int. J. Mol. Sci. 2021, 22, 359. https://doi.org/10.3390/ijms22010359
Neroev V, Malishevskaya T, Weinert D, Astakhov S, Kolomeichuk S, Cornelissen G, Kabitskaya Y, Boiko E, Nemtsova I, Gubin D. Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma. International Journal of Molecular Sciences. 2021; 22(1):359. https://doi.org/10.3390/ijms22010359
Chicago/Turabian StyleNeroev, Vladimir, Tatyana Malishevskaya, Dietmar Weinert, Sergei Astakhov, Sergey Kolomeichuk, Germaine Cornelissen, Yana Kabitskaya, Elena Boiko, Irina Nemtsova, and Denis Gubin. 2021. "Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma" International Journal of Molecular Sciences 22, no. 1: 359. https://doi.org/10.3390/ijms22010359
APA StyleNeroev, V., Malishevskaya, T., Weinert, D., Astakhov, S., Kolomeichuk, S., Cornelissen, G., Kabitskaya, Y., Boiko, E., Nemtsova, I., & Gubin, D. (2021). Disruption of 24-Hour Rhythm in Intraocular Pressure Correlates with Retinal Ganglion Cell Loss in Glaucoma. International Journal of Molecular Sciences, 22(1), 359. https://doi.org/10.3390/ijms22010359