Choline Acetyltransferase Induces the Functional Regeneration of the Salivary Gland in Aging SAMP1/Kl -/- Mice
Abstract
:1. Introduction
2. Results
2.1. Metabolic Profiling of Salivary Glands in SAMP1/kl +/+ and SAMP1 kl -/- Mice
2.2. Saliva Secretion Mediated by Acetylcholine (Ach) in SAMP1/kl -/- Mice
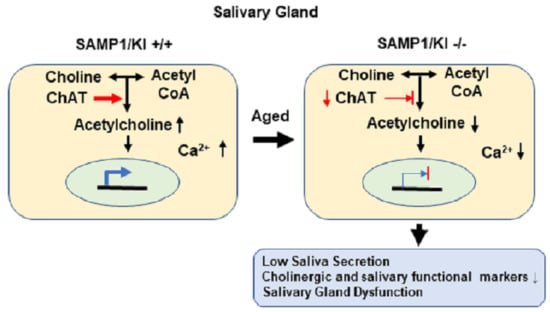
2.3. Choline Acetyltransferase (ChAT) is Downregulated in Primary Salivary Gland (PSGC) kl -/- Cells and SAMP1/kl -/- Mice
2.4. ChAT Regulates the Expression of Salivary Gland Functional Genes
2.5. Knockdown of ChAT Inhibits Salivary Gland Function
2.6. In Vivo Salivary Gland Transduction Using Adeno-Associated Virus (AAV) Vectors Expressing ChAT
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Metabolome Profile Analysis of Mouse Submandibular Salivary Gland Tissue by Capillary Electrophoresis Time-of-Flight Mass Spectrometry (CE-TOFMS) Analysis
4.3. Data Processing and Analysis
4.4. Biochemistry Analysis
4.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.6. Transfection Assay
4.7. Western Blot Analysis
4.8. Viral Vector Delivery and Immunohistochemistry Staining
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, J. Structure and Function in Aging Human Salivary Glands. Gerodontology 1986, 5, 149–158. [Google Scholar] [CrossRef]
- Scott, J.; Flower, E.A.; Burns, J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J. Oral Pathol. 1987, 16, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.D. The role of saliva in maintaining oral homeostasis. J. Am. Dent. Assoc. 1989, 119, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Spijkervet, F.K.; Van Nieuw Amerongen, A. Aging and saliva: A review of the literature. Spec. Care Dent. 1996, 16, 95–103. [Google Scholar] [CrossRef]
- Abiko, Y.; Nishimura, M.; Kaku, T. Defensins in saliva and the salivary glands. Med. Electron. Microsc. 2003, 36, 247–252. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Ładny, J.R. Salivary Antioxidant Barrier, Redox Status, and Oxidative Damage to Proteins and Lipids in Healthy Children, Adults, and the Elderly. Oxid. Med. Cell. Longev. 2019, 2019, 4393460. [Google Scholar] [CrossRef] [Green Version]
- Pushpass, R.G.; Daly, B.; Kelly, C.; Proctor, G.; Carpenter, G.H. Altered Salivary Flow, Protein Composition, and Rheology Following Taste and TRP Stimulation in Older Adults. Front. Physiol. 2019, 10, 652. [Google Scholar] [CrossRef]
- Ship, J.A.; Baum, B.J. Is reduced salivary flow normal in old people? Lancet 1990, 336, 1507. [Google Scholar] [CrossRef]
- Dodds, M.W.; Johnson, D.A.; Yeh, C.K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Zussman, E.; Yarin, A.L.; Nagler, R.M. Age- and flow-dependency of salivary viscoelasticity. J. Dent. Res. 2007, 86, 281–285. [Google Scholar] [CrossRef]
- Bourdiol, P.; Mioche, L.; Monier, S. Effect of age on salivary flow obtained under feeding and non-feeding conditions. J. Oral Rehabil. 2004, 31, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.W.; Chung, J.W.; Kho, H.S.; Chung, S.C.; Kim, Y.K. Nerve growth factor concentration in human saliva. Oral Dis. 2007, 13, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Yeh, C.K.; Dodds, M.W. Effect of donor age on the concentrations of histatins in human parotid and submandibular/sublingual saliva. Arch. Oral Biol. 2000, 45, 731–740. [Google Scholar] [CrossRef]
- Scott, J. Structural Age Changes in Salivary Glands. Front. Oral Biol. 1987, 6, 40–62. [Google Scholar]
- Kwon, S.M.; Kim, S.A.; Yoon, J.H.; Yook, J.I.; Ahn, S.G. Global analysis of gene expression profiles in the submandibular salivary gland of klotho knockout mice. J. Cell. Physiol. 2018, 233, 3282–3294. [Google Scholar] [CrossRef] [Green Version]
- Tai, N.C.; Kim, S.A.; Ahn, S.G. Soluble klotho regulates the function of salivary glands by activating KLF4 pathways. Aging (Albany NY) 2019, 11, 8254–8269. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Suzuki, H.; Amizuka, N.; Noda, M.; Amano, O.; Maeda, T. Histological and immunohistochemical changes in the submandibular gland in klotho-deficient mice. Arch. Histol. Cytol. 2006, 69, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Yahata, K.; Mukoyama, M.; Suganami, T.; Makino, H.; Nagae, T.; Masuzaki, H.; Ogawa, Y.; Sugawara, A.; Nabeshima, Y.-i.; et al. Disruption of klotho gene causes an abnormal energy homeostasis in mice. Biochem. Biophys. Res. Commun. 2000, 278, 665–670. [Google Scholar] [CrossRef]
- Kim, S.A.; Lam, T.G.; Yook, J.I.; Ahn, S.G. Antioxidant modifications induced by the new metformin derivative HL156A regulate metabolic reprogramming in SAMP1/kl (-/-) mice. Aging (Albany NY) 2018, 10, 2338–2355. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, G.B. The physiology of salivary secretion. Periodontology 2000 2016, 70, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isola, G.; Polizzi, A.; Patini, R.; Ferlito, S.; Alibrandi, A.; Palazzo, G. Association among serum and salivary A. actinomycetemcomitans specific immunoglobulin antibodies and periodontitis. BMC Oral Health 2020, 20, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020, 1–11. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Polizzi, A.; Alibrandi, A.; Murabito, P.; Indelicato, F. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch. Oral Biol. 2021, 122, 104997. [Google Scholar] [CrossRef]
- Park, D.; Yang, Y.-H.; Bae, D.K.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Choi, E.-K.; Lee, S.W.; Kim, G.H.; et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol. Aging 2013, 34, 2639–2646. [Google Scholar] [CrossRef]
- Coppes, R.P.; Zeilstra, L.J.; Kampinga, H.H.; Konings, A.W. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br. J. Cancer 2001, 85, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Baum, B.J.; Alevizos, I.; Zheng, C.; Cotrim, A.P.; Liu, S.; McCullagh, L.; Goldsmith, C.M.; Burbelo, P.D.; Citrin, D.E.; Mitchell, J.B.; et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc. Natl. Acad. Sci. USA 2012, 109, 19403–19407. [Google Scholar] [CrossRef] [Green Version]
- Quirin-Stricker, C.; Mauvais, C.; Schmitt, M. Transcriptional activation of human choline acetyltransferase by AP2- and NGF-induced factors. Brain Res. Mol. Brain Res. 1997, 49, 165–174. [Google Scholar] [CrossRef]
- Madziar, B.; Lopez-Coviella, I.; Zemelko, V.; Berse, B. Regulation of cholinergic gene expression by nerve growth factor depends on the phosphatidylinositol-3’-kinase pathway. J. Neurochem. 2005, 92, 767–779. [Google Scholar] [CrossRef] [PubMed]






| Gene | Sequence |
|---|---|
| M1AchR | Forward: 5’– TCTCTGAATGCTGGAAGTAAAGA – 3’ Reverse: 5’– GAGACCCTAGATTCAGTCCCA – 3’ |
| M3AchR | Forward: 5’– AGGGCTGACTACTTAATCTTGGATA – 3’ Reverse: 5’– TGCAAGGTCATTGTGACTCTC – 3’ |
| ATP2α1 | Forward: 5’– GAGCAGTTCGAAGACCTGCTTGTG – 3’ Reverse: 5’– CCTGTCAGGATGGACTGGTCGA – 3’ |
| ChAT | Forward: 5’– GTTATAACCCCCAGCCTGAGGCC – 3’ Reverse: 5’– GGTCTCTCATGTCAACAAGGCTCGC – 3’ |
| α-Amylase | Forward: 5’– GGTGCAACAATGTTGGTGTC – 3’ Reverse: 5’– ACTGCTTTGTCCAGCTTGAG – 3’ |
| Zo-1 | Forward: 5’– CGAGGCATCATCCCAAATAAGAAC – 3’ Reverse: 5’– TCCAGAAGTCTGCCCGATCAC – 3’ |
| Aqp5 | Forward: 5’– CGACCGTGTGGCTGTGGTCA – 3’ Reverse: 5’– GTGCCGGTCAGTGTGCCGTC – 3’ |
| GAPDH | Forward: 5’– AGCCAAAAGGGTCATCATCTCTGC – 3’ Reverse: 5’– CCTTCCACAATGCCAAAGTTGTCA – 3’ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toan, N.K.; Tai, N.C.; Kim, S.-A.; Ahn, S.-G. Choline Acetyltransferase Induces the Functional Regeneration of the Salivary Gland in Aging SAMP1/Kl -/- Mice. Int. J. Mol. Sci. 2021, 22, 404. https://doi.org/10.3390/ijms22010404
Toan NK, Tai NC, Kim S-A, Ahn S-G. Choline Acetyltransferase Induces the Functional Regeneration of the Salivary Gland in Aging SAMP1/Kl -/- Mice. International Journal of Molecular Sciences. 2021; 22(1):404. https://doi.org/10.3390/ijms22010404
Chicago/Turabian StyleToan, Nguyen Khanh, Nguyen Chi Tai, Soo-A Kim, and Sang-Gun Ahn. 2021. "Choline Acetyltransferase Induces the Functional Regeneration of the Salivary Gland in Aging SAMP1/Kl -/- Mice" International Journal of Molecular Sciences 22, no. 1: 404. https://doi.org/10.3390/ijms22010404
APA StyleToan, N. K., Tai, N. C., Kim, S. -A., & Ahn, S. -G. (2021). Choline Acetyltransferase Induces the Functional Regeneration of the Salivary Gland in Aging SAMP1/Kl -/- Mice. International Journal of Molecular Sciences, 22(1), 404. https://doi.org/10.3390/ijms22010404