Time-Lapse Flow Cytometry: A Robust Tool to Assess Physiological Parameters Related to the Fertilizing Capability of Human Sperm
Abstract
:1. Introduction
2. Results
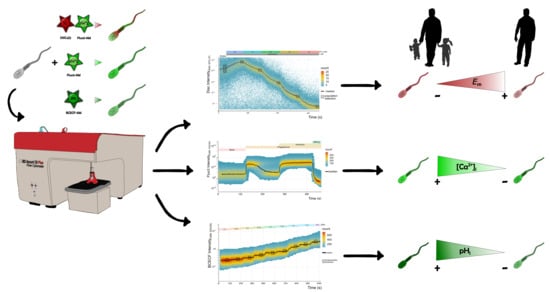
2.1. Measurement of Em Absolute Values in Human Sperm Using TLFC Recordings
2.2. Simultaneous Measurement of Em and Evaluation of [Ca2+]i Changes in Human Sperm Using TLFC Recordings
2.3. Qualitative Analysis of [Ca2+]i Changes in Human Sperm Using TLFC Recordings
2.4. Measurement of pHi Absolute Values in Human Sperm Using TLFC
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Human Sperm Samples and Ethical Approval
4.3. Human Sperm Sample Preparation
4.4. Experimental Setup to Perform TLFC
4.5. Data Acquisition and Selection of Viable Single Sperm Cells in the BD Accuri C6 Plus Flow Cytometer
4.6. Measurement of Absolute Em Values in Human Sperm
4.7. Evaluation of [Ca2+]i Changes in Human Sperm
4.8. Measurement of Absolute pHi Values in Human Sperm
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| [Ca2+]i | Intracellular calcium concentration |
| AR | Acrosomal reaction |
| ART | Artificial reproduction techniques |
| BCECF | BCECF-AM |
| BSA | Bovine serum albumin |
| CAP | Capacitating |
| CSV | comma-separated values |
| Disc | DiSC3(5) |
| DMSO | Dimethyl sulfoxide |
| Em | Membrane potential |
| Fluo3 | Fluo3-AM |
| FCS | Flow Cytometry Standard |
| H+Cal | Calibration medium |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HTF | Human tubal fluid |
| Iono | Ionomycin |
| Mn2+ | Manganese chloride |
| NC | Non-capacitating |
| Pg | Progesterone |
| pHe | Extracellular pH |
| pHi | Intracellular pH |
| PM | Plasma membrane |
| SEM | Standard error of the mean |
| TLFC | Time-lapse flow cytometry |
| WHO | World Health Organization |
References
- Sharma, R.; Biedenharn, K.R.; Fedor, J.M.; Agarwal, A. Lifestyle factors and reproductive health: Taking control of your fertility. Reprod. Biol. Endocrinol. 2013, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HFEA. Fertility Treatment 2014. Trends and Figures. Available online: https://www.hfea.gov.uk/media/1111/hfea-fertility-treatment-trends-and-figures-2014.pdf (accessed on 27 November 2020).
- Kumar, N.; Singh, A. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Martini, F.O.; McLachlan, R.; Oates, R.D.; van der Poel, S.; John, B.S.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Ford, W.C.L. Comments on the release of the 5th edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J. 2010, 12, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.G.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef]
- MacLeod, J. The Male Factor in Fertility and Infertility: An Analysis of Ejaculate Volume in 800 Fertile Men and in 600 Men in Infertile Marriage. Fertil. Steril. 1950, 1, 347–361. [Google Scholar] [CrossRef]
- Björndahl, L. What is normal semen quality? On the use and abuse of reference limits for the interpretation of semen analysis results. Hum. Fertil. 2011, 14, 179–186. [Google Scholar] [CrossRef]
- Bracke, A.; Peeters, K.; Punjabi, U.; Hoogewijs, D.; Dewilde, S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod. Biomed. Online 2018, 36, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Tosti, E.; Ménézo, Y. Gamete activation: Basic knowledge and clinical applications. Hum. Reprod. Update 2016, 22, 420–439. [Google Scholar] [CrossRef] [Green Version]
- Chang, M. Fertilizing Capacity of Spermatozoa deposited into the Fallopian Tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Biol. Sci. 1951, 4, 581–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puga Molina, L.C.; Luque, G.M.; Balestrini, P.A.; Marín-Briggiler, C.I.; Romarowski, A.; Buffone, M.G. Molecular Basis of Human Sperm Capacitation. Front. Cell Dev. Biol. 2018, 6, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Gonzalez, I.; Torres-Rodriguez, P.; Sanchez-Carranza, O.; Solis-Lopez, A.; Santi, C.M.; Darszon, A.; Trevino, C.L. Membrane hyperpolarization during human sperm capacitation. Mol. Hum. Reprod. 2014, 20, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baro Graf, C.; Ritagliati, C.; Stival, C.; Balestrini, P.A.; Buffone, M.G.; Krapf, D. Determination of a robust assay for human sperm membrane potential analysis. Front. Cell Dev. Biol. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Zeng, Y.; Clark, E.N.; Florman, H.M. Sperm membrane potential: Hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 1995, 171, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Tosti, E.; Boni, R. Electrical events during gamete maturation and fertilization in animals and humans. Hum. Reprod. Update 2004, 10, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Matamoros-Volante, A.; Treviño, C.L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Cross, N.L.; Razy-Faulkner, P. Control of human sperm intracellular pH by cholesterol and its relationship to the response of the acrosome to progesterone. Biol. Reprod. 1997, 56, 1169–1174. [Google Scholar] [CrossRef]
- Hamamah, S.; Magnoux, E.; Royere, D.; Barthelemy, C.; Dacheux, J.L.; Gatti, J.L. Internal pH of human spermatozoa: Effect of ions, human follicular fluid and progesterone. Mol. Hum. Reprod. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P. V Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Strünker, T.; Goodwin, N.; Brenker, C.; Kashikar, N.D.; Weyand, I.; Seifert, R.; Kaupp, U.B. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature 2011, 471, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Mansell, S.A.; Lishko, P.V.; Williams, H.L.; Ramalingam, M.; Wilson, S.M.; Barratt, C.L.R.; Sutton, K.A.; Da Silva, S.M. Depolarization of sperm membrane potential is a common feature of men with subfertility and is associated with low fertilization rate at IVF. Hum. Reprod. 2016, 31, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Baro Graf, C.; Ritagliati, C.; Torres-Monserrat, V.; Stival, C.; Carizza, C.; Buffone, M.G.; Krapf, D. Membrane Potential Assessment by Fluorimetry as a Predictor Tool of Human Sperm Fertilizing Capacity. Front. Cell Dev. Biol. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Puga-Molina, L.C.P.; Gunderson, S.; Riley, J.; Lybaert, P.; Borrego-Alvarez, A.; Jungheim, E.S.; Santi, C.M. Membrane Potential Determined by Flow Cytometry Predicts Fertilizing Ability of Human Sperm. Front. Cell Dev. Biol. 2020, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, S.; Puga Molina, L.C.; Riley, J.; Jungheim, E.S.; Santi, C.M. Sperm intracellular pH as a predictor of fertilization rate in normospermic infertile men undergoing in vitro fertilization. Fertil. Steril. 2019, 112, e201–e202. [Google Scholar] [CrossRef]
- Kelly, M.C.; Brown, S.G.; Costello, S.M.; Ramalingam, M.; Drew, E.; Publicover, S.J.; Barratt, C.L.R.; Da Silva, S.M. Single-cell analysis of [Ca2+]i signalling in sub-fertile men: Characteristics and relation to fertilization outcome. Hum. Reprod. 2018, 33, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Krausz, C.; Bonaccorsi, L.; Luconi, M.; Fuzzi, B.; Criscuoli, L.; Pellegrini, S.; Forti, G.; Baldi, E. Intracellular calcium increase and acrosome reaction in response to progesterone in human spermatozoa are correlated with in-vitro fertilization. Hum. Reprod. 1995, 10, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Darszon, A.; Nishigaki, T.; Wood, C.; Treviño, C.L.; Felix, R.; Beltrán, C. Calcium channels and Ca2+ fluctuations in sperm physiology. Int. Rev. Cytol. 2005, 243, 79–172. [Google Scholar] [CrossRef]
- Kirichok, Y.; Lishko, P.V. Rediscovering sperm ion channels with the patch-clamp technique. Mol. Hum. Reprod. 2011, 17, 478–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vines, A.; McBean, G.J.; Blanco-Fernández, A. A flow-cytometric method for continuous measurement of intracellular Ca2+ concentration. Cytom. Part A 2010, 77A, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Franchi, A.; Moreno-Irusta, A.; Domínguez, E.M.; Adre, A.J.; Giojalas, L.C. Extracellular vesicles from oviductal isthmus and ampulla stimulate the induced acrosome reaction and signaling events associated with capacitation in bovine spermatozoa. J. Cell. Biochem. 2020, 121, 2877–2888. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Kirichok, Y.; Clapham, D.E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. USA 2007, 104, 7688–7692. [Google Scholar] [CrossRef] [Green Version]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Plášek, J.; Hrouda, V. Assessment of membrane potential changes using the carbocyanine dye, diS-C3-(5): Synchronous excitation spectroscopy studies. Eur. Biophys. J. 1991, 19, 183–188. [Google Scholar] [CrossRef]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Trevino, C.L. Calcium Channels in the Development, Maturation, and Function of Spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [Green Version]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.J.; Clapham, D.E. The control of male fertility by spermatozoan ion channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef] [Green Version]
- Darszon, A.; Nishigaki, T.; López-González, I.; Visconti, P.E.; Treviño, C.L. Differences and similarities: The richness of comparative sperm physiology. Physiology 2020, 35, 196–208. [Google Scholar] [CrossRef]
- Avenarius, M.R.; Hildebrand, M.S.; Zhang, Y.; Meyer, N.C.; Smith, L.L.H.; Kahrizi, K.; Najmabadi, H.; Smith, R.J.H. Human Male Infertility Caused by Mutations in the CATSPER1 Channel Protein. Am. J. Hum. Genet. 2009, 84, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Avidan, N.; Tamary, H.; Dgany, O.; Cattan, D.; Pariente, A.; Thulliez, M.; Borot, N.; Moati, L.; Barthelme, A.; Shalmon, L.; et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur. J. Hum. Genet. 2003, 11, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkman-Brown, J.C.; Bray, C.; Stewart, P.M.; Barratt, C.L.R.; Publicover, S.J. Biphasic elevation of [Ca2+](i) in individual human spermatozoa exposed to progesterone. Dev. Biol. 2000, 222, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mata-Martínez, E.; José, O.; Torres-Rodríguez, P.; Solís-López, A.; Sánchez-Tusie, A.A.; Sánchez-Guevara, Y.; Treviño, M.B.; Treviño, C.L. Measuring intracellular Ca2+ changes in human sperm using four techniques: Conventional fluorometry, stopped flow fluorometry, flow cytometry and single cell imaging. J. Vis. Exp. 2013, e50344. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Kumar, A.; Swain, D.K.; Yadav, S.; Nigam, R. Insights into pH regulatory mechanisms in mediating spermatozoa functions. Vet. World 2018, 11, 852–858. [Google Scholar] [CrossRef]
- Nishigaki, T.; José, O.; González-Cota, A.L.; Romero, F.; Treviño, C.L.; Darszon, A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar] [CrossRef] [Green Version]
- Chávez, J.C.; Darszon, A.; Treviño, C.L.; Nishigaki, T. Quantitative Intracellular pH Determinations in Single Live Mammalian Spermatozoa Using the Ratiometric Dye SNARF-5F. Front. Cell Dev. Biol. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Patrat, C.; Serres, C.; Jouannet, P. Progesterone induces hyperpolarization after a transient depolarization phase in human spermatozoa. Biol. Reprod. 2002, 66, 1775–1780. [Google Scholar] [CrossRef] [Green Version]
- Brewis, I.A.; Morton, I.E.; Mohammad, S.N.; Browes, C.E.; Moore, H.D.M. Measurement of intracellular calcium concentration and plasma membrane potential in human spermatozoa using flow cytometry. J. Androl. 2000, 21, 238–249. [Google Scholar] [CrossRef]
- Baldi, E.; Casano, R.; Falsetti, C.; Krausz, C.; Maggi, M.; Forti, G. Intracellular Calcium Accumulation and Responsiveness to Progesterone in Capacitating Human Spermatozoa. J. Androl. 1991, 12, 323–330. [Google Scholar] [CrossRef]
- Garcia, M.A.; Meizel, S. Progesterone-Mediated Calcium Influx and Acrosome Reaction of Human Spermatozoa: Pharmacological Investigation of T-Type Calcium Channels1. Biol. Reprod. 1999, 60, 102–109. [Google Scholar] [CrossRef] [Green Version]
- González-Martínez, M.T.; Bonilla-Hernández, M.A.; Guzmán-Grenfell, A.M. Stimulation of voltage-dependent calcium channels during capacitation and by progesterone in human sperm. Arch. Biochem. Biophys. 2002, 408, 205–210. [Google Scholar] [CrossRef]
- Mendoza, C.; Tesarik, J. A plasma-membrane progesterone receptor in human sperm is switched on by increasing intracellular free calcium. FEBS Lett. 1993, 330, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Torres-Flores, V.; Hernandez-Rueda, Y.L.; del Carmen Neri-Vidaurri, P.; Jimenez-Trejo, F.; Calderon-Salinas, V.; Molina-Guarneros, J.A.; Gonzalez-Martinez, M.T. Activation of Protein Kinase A Stimulates the Progesterone-Induced Calcium Influx in Human Sperm Exposed to the Phosphodiesterase Inhibitor Papaverine. J. Androl. 2008, 29, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nord, E.P.; Bronson, R.A. Progesterone-evoked increases in sperm [Ca2+]i correlate with the egg penetrating ability of sperm from fertile but not infertile men. Fertil. Steril. 1993, 60, 526–532. [Google Scholar] [CrossRef]
- Krausz, C.; Bonaccorsi, L.; Maggio, P.; Luconi, M.; Criscuoli, L.; Fuzzi, B.; Pellegrini, S.; Forti, G.; Baldi, E. Two functional assays of sperm responsiveness to progesterone and their predictive values in in-vitro fertilization. Hum. Reprod. 1996, 11, 1661–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falsetti, C.; Baldi, E.; Krausz, C.; Casano, R.; Failli, P.; Forti, G. Decreased Responsiveness to Progesterone of Spermatozoa in Oligozoospermic Patients. J. Androl. 1993, 14, 17–22. [Google Scholar] [CrossRef]
- Guzmán-Grenfell, A.M.; González-Martínez, M.T. Lack of Voltage-Dependent Calcium Channel Opening During the Calcium Influx Induced by Progesterone in Human Sperm. Effect of Calcium Channel Deactivation and Inactivation. J. Androl. 2004, 25, 117–122. [Google Scholar] [CrossRef]
- Linares-Hernández, L.; Guzmán-Grenfell, A.M.; Hicks-Gomez, J.J.; González-Martínez, M.T. Voltage-dependent calcium influx in human sperm assessed by simultaneous optical detection of intracellular calcium and membrane potential. Biochim. Biophys. Acta (BBA)-Biomembr. 1998, 1372, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Kirichok, Y.; Navarro, B.; Clapham, D.E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 2006, 439, 737–740. [Google Scholar] [CrossRef]
- Balestrini, P.A.; Jabloñski, M.; Schiavi-Ehrenhaus, L.J.; Marín-Briggiler, C.I.; Sánchez-Cárdenas, C.; Darszon, A.; Krapf, D.; Buffone, M.G. Seeing is believing: Current methods to observe sperm acrosomal exocytosis in real time. Mol. Reprod. Dev. 2020. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Zheng, J.; Lin, J.; Zhang, L.; Jin, J. The progesterone-induced sperm acrosome reaction is a good option for the prediction of fertilization in vitro compared with other sperm parameters. Andrologia 2019, 51. [Google Scholar] [CrossRef] [PubMed]
- Lachance, C.; Goupil, S.; Tremblay, R.R.; Leclerc, P. The immobilization of human spermatozoa by STAT3 inhibitory compound V results from an excessive intracellular amount of reactive oxygen species. Andrology 2016, 4, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Puga Molina, L.C.; Pinto, N.A.; Torres Rodríguez, P.; Romarowski, A.; Vicens Sanchez, A.; Visconti, P.E.; Darszon, A.; Treviño, C.L.; Buffone, M.G. Essential Role of CFTR in PKA-Dependent Phosphorylation, Alkalinization, and Hyperpolarization During Human Sperm Capacitation. J. Cell. Physiol. 2017, 232, 1404–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team. RStudio: Integrated Development Environment for R 2020; RStudio Team: Bostom, MA, USA, 2020. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; ISBN 978-0-387-98140-6. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matamoros-Volante, A.; Castillo-Viveros, V.; Torres-Rodríguez, P.; Treviño, M.B.; Treviño, C.L. Time-Lapse Flow Cytometry: A Robust Tool to Assess Physiological Parameters Related to the Fertilizing Capability of Human Sperm. Int. J. Mol. Sci. 2021, 22, 93. https://doi.org/10.3390/ijms22010093
Matamoros-Volante A, Castillo-Viveros V, Torres-Rodríguez P, Treviño MB, Treviño CL. Time-Lapse Flow Cytometry: A Robust Tool to Assess Physiological Parameters Related to the Fertilizing Capability of Human Sperm. International Journal of Molecular Sciences. 2021; 22(1):93. https://doi.org/10.3390/ijms22010093
Chicago/Turabian StyleMatamoros-Volante, Arturo, Valeria Castillo-Viveros, Paulina Torres-Rodríguez, Marcela B. Treviño, and Claudia L. Treviño. 2021. "Time-Lapse Flow Cytometry: A Robust Tool to Assess Physiological Parameters Related to the Fertilizing Capability of Human Sperm" International Journal of Molecular Sciences 22, no. 1: 93. https://doi.org/10.3390/ijms22010093
APA StyleMatamoros-Volante, A., Castillo-Viveros, V., Torres-Rodríguez, P., Treviño, M. B., & Treviño, C. L. (2021). Time-Lapse Flow Cytometry: A Robust Tool to Assess Physiological Parameters Related to the Fertilizing Capability of Human Sperm. International Journal of Molecular Sciences, 22(1), 93. https://doi.org/10.3390/ijms22010093