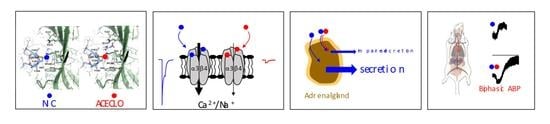
Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure
Abstract
:1. Introduction
2. Results
2.1. Effect of Neonicotinoids on ACh-Evoked Cholinergic Currents
2.2. Effects of Neonicotinoids on Epinephrine Secretion in Rat
2.3. Effects of Neonicotinoids on ABP in Rat
2.4. Modeling of Neonicotinoid–34AChR Interaction
3. Discussion
4. Materials and Methods
4.1. Animal Models and Experimental Conditions
4.2. Solutions and Drugs
4.3. Molecular Cloning of Rat nAChR Subunits
4.4. Expression of 34AChRs in Xenopus Oocytes
4.5. Two-Electrode Voltage Clamp Recording
4.6. Epinephrine Secretion Assay
4.7. Intoxication Protocol and ABP Measurement
4.8. nAChR Homology Modeling and Ligand Docking
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | arterial blood pressure |
| ACE | acetamiprid |
| ACh | acetylcholine |
| AngII | angiotensin II |
| CLO | clothianidin |
| DMSO | dimethyl sulfoxide |
| EC | half maximal effective concentration |
| HEX | hexamethonium |
| IMI | imidacloprid |
| nAChR | nicotinic acetylcholine receptor |
| NIC | nicotine |
| VEH | vehicle |
References
- Tomizawa, M.; Casida, J.E. Neonicotinoid Insecticide Toxicology: Mechanisms of Selective Action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef]
- Douglas, M.R.; Tooker, J.F. Large-Scale Deployment of Seed Treatments Has Driven Rapid Increase in Use of Neonicotinoid Insecticides and Preemptive Pest Management in U.S. Field Crops. Environ. Sci. Technol. 2015, 49, 5088–5097. [Google Scholar] [CrossRef] [PubMed]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, S.D.; Tooker, J.F. Opinion: Neonicotinoids pose undocumented threats to food webs. Proc. Natl. Acad. Sci. USA 2020, 117, 202017221. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef]
- Mörtl, M.; Vehovszky, A.; Klátyik, S.; Takács, E.; Győri, J.; Székács, A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [Green Version]
- Pisa, L.; Goulson, D.; Yang, E.C.; Gibbons, D.; Sánchez-Bayo, F.; Mitchell, E.; Aebi, A.; Sluijs, J.v.d.; MacQuarrie, C.J.K.; Giorio, C.; et al. An update of the Worldwide Integrated Assessment (WIA) on systemic insecticides. Part 2: Impacts on organisms and ecosystems. Environ. Sci. Pollut. Res. 2017, 28, 11749–11797. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Anadón, A.; Qinghua, W.; Qiao, F.; Ares, I.; Martínez-Larrañaga, M.R.; Yuan, Z.; Martínez, M.A. Mechanism of Neonicotinoid Toxicity: Impact on Oxidative Stress and Metabolism. Annu. Rev. Pharmacol. Toxicol. 2017, 58, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, N.; Hirano, T.; Omotehara, T.; Tokumoto, J.; Umemura, Y.; Mantani, Y.; Tanida, T.; Warita, K.; Tabuchi, Y.; Yokoyama, T.; et al. Insight into the Mechanism of Reproductive Dysfunction Caused by Neonicotinoid Pesticides. Biol. Pharm. Bull. 2014, 37, 1439–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Mateo, R. Imidacloprid-treated seed ingestion has lethal effect on adult partridges and reduces both breeding investment and offspring immunity. Environ. Res. 2015, 136, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.R.; Li, S.; Zhang, J.; Liang, C.; Chen, E.N.; Zhang, S.Y.; Chuai, M.; Bao, Y.P.; Wang, G.; Yang, X. Excess Imidacloprid Exposure Causes the Heart Tube Malformation of Chick Embryos. J. Agric. Food Chem. 2016, 64, 9078–9088. [Google Scholar] [CrossRef] [Green Version]
- Gobeli, A.; Crossley, D.; Johnson, J.; Reyna, K. The effects of neonicotinoid exposure on embryonic development and organ mass in northern bobwhite quail (Colinus virginianus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 9–15. [Google Scholar] [CrossRef]
- Ge, W.; Yan, S.; Wang, J.; Zhu, L.; Chen, A.; Wang, J. Oxidative Stress and DNA Damage Induced by Imidacloprid in Zebrafish (Danio rerio). J. Agric. Food Chem. 2015, 63, 1856–1862. [Google Scholar] [CrossRef]
- Ansoar-Rodríguez, Y.; Christofoletti, C.A.; Correia, J.E.; Souza, R.B.D.; Moreira-de Sousa, C.; Marcato, A.C.d.C.; Bueno, O.C.; Malaspina, O.; Silva-Zacarin, E.C.M.; Fontanetti, C.S. Liver alterations in Oreochromis niloticus (Pisces) induced by insecticide imidacloprid: Histopathology and heat shock protein in situ localization. J. Environ. Sci. Health Part B 2016, 51, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Iturburu, F.G.; Zömisch, M.; Panzeri, A.M.; Crupkin, A.C.; Contardo-Jara, V.; Pflugmacher, S.; Menone, M.L. Uptake, distribution in different tissues, and genotoxicity of imidacloprid in the freshwater fish Australoheros facetus. Environ. Toxicol. Chem. 2017, 36, 699–708. [Google Scholar] [CrossRef]
- Hirano, T.; Yanai, S.; Omotehara, T.; Hashimoto, R.; Umemura, Y.; Kubota, N.; Minami, K.; Nagahara, D.; Matsuo, E.; Aihara, Y.; et al. The combined effect of clothianidin and environmental stress on the behavioral and reproductive function in male mice. J. Vet. Med. Sci. 2015, 77, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, C.J.; Lin, C.L.; Lin, T.Y.; Wang, S.E.; Wu, C.H. Imidacloprid toxicity impairs spatial memory of echolocation bats through neural apoptosis in hippocampal CA1 and medial entorhinal cortex areas. NeuroReport 2016, 27, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Niibori, Y.; Terayama, H.; Ito, M.; Pidgeon, C.; Arsenault, J.; Camarero, P.R.; Cummins, C.L.; Mateo, R.; Sakabe, K.; et al. Mammalian Susceptibility to a Neonicotinoid Insecticide after Fetal and Early Postnatal Exposure. Sci. Rep. 2018, 8, 16639. [Google Scholar] [CrossRef] [Green Version]
- Berheim, E.H.; Jenks, J.A.; Lundgren, J.G.; Michel, E.S.; Grove, D.; Jensen, W.F. Effects of Neonicotinoid Insecticides on Physiology and Reproductive Characteristics of Captive Female and Fawn White-tailed Deer. Sci. Rep. 2019, 9, 4534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomizawa, M.; Kagabu, S.; Casida, J.E. Receptor Structure-Guided Neonicotinoid Design. J. Agric. Food Chem. 2011, 59, 2918–2922. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tao, L.; McLean, J.; Lu, C. Quantitative Analysis of Neonicotinoid Insecticide Residues in Foods: Implication for Dietary Exposures. J. Agric. Food Chem. 2014, 62, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, Y.; Lv, B.; Liu, Z.; Han, J.; Li, J.; Zhao, Y.; Wu, Y. Dietary exposure to neonicotinoid insecticides and health risks in the Chinese general population through two consecutive total diet studies. Environ. Int. 2020, 135, 105399. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chang, C.H.; Palmer, C.; Zhao, M.; Zhang, Q. Neonicotinoid Residues in Fruits and Vegetables: An Integrated Dietary Exposure Assessment Approach. Environ. Sci. Technol. 2018, 52, 3175–3184. [Google Scholar] [CrossRef]
- Tao, Y.; Phung, D.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Liu, Q.; He, M.; Pan, X.; Li, R.; et al. Urinary monitoring of neonicotinoid imidacloprid exposure to pesticide applicators. Sci. Total Environ. 2019, 669, 721–728. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Chang, C.H.; Yu, C.; Wang, X.; Lu, C. Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ. Int. 2019, 126, 672–681. [Google Scholar] [CrossRef]
- Lin, P.; Lin, H.; Liao, Y.; Guo, H.; Chen, K. Acute Poisoning with Neonicotinoid Insecticides: A Case Report and Literature Review. Basic Clin. Pharmacol. Toxicol. 2013, 112, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2016, 125, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.; Grobman, B.; Krishnan-Sarin, S. Recent findings in the pharmacology of inhaled nicotine: Preclinical and clinical in vivo studies. Neuropharmacology 2020, 176, 108218. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutkiewicz, E.M.; Rice, K.C.; Carroll, F.I.; Woods, J.H. Patterns of nicotinic receptor antagonism II: Cardiovascular effects in rats. Drug Alcohol Depend. 2013, 131, 284–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millar, N.S.; Denholm, I. Nicotinic acetylcholine receptors: Targets for commercially important insecticides. Invertebr. Neurosci. 2007, 7, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Schmeltz, I. Nicotine and Other Tobacco Alkaloids; Marcel Dekker: New York, NY, USA, 1971. [Google Scholar]
- Xiao, Y.; Meyer, E.L.; Thompson, J.M.; Surin, A.; Wroblewski, J.; Kellar, K.J. Rat α3β4 Subtype of Neuronal Nicotinic Acetylcholine Receptor Stably Expressed in a Transfected Cell Line: Pharmacology of Ligand Binding and Function. Mol. Pharmacol. 1998, 54, 322–333. [Google Scholar] [CrossRef]
- Li, P.; Ann, J.; Akk, G. Activation and modulation of human α4β2 nicotinic acetylcholine receptors by the neonicotinoids clothianidin and imidacloprid. J. Neurosci. Res. 2011, 89, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Cartereau, A.; Martin, C.; Thany, S.H. Neonicotinoid insecticides differently modulate acetycholine-induced currents on mammalian α7 nicotinic acetylcholine receptors. Br. J. Pharmacol. 2018, 175, 1987–1998. [Google Scholar] [CrossRef]
- Free, R.; Bryant, D.L.; McKay, S.B.; Kaser, D.J.; McKay, D.B. [3H]Epibatidine binding to bovine adrenal medulla: Evidence for α3β4* nicotinic receptors. Neurosci. Lett. 2002, 318, 98–102. [Google Scholar] [CrossRef]
- De Nardi, F.; Lefort, C.; Bréard, D.; Richomme, P.; Legros, C.; Guérineau, N.C. Monitoring the Secretory Behavior of the Rat Adrenal Medulla by High-Performance Liquid Chromatography-Based Catecholamine Assay from Slice Supernatants. Front. Endocrinol. 2017, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Nistri, A.; Cavalli, A.; Carloni, P. A structural model of agonist binding to the alpha3beta4 neuronal nicotinic receptor. Br. J. Pharmacol. 2003, 140, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lindstrom, J. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2017, 175, 1805–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and Blood Pressure Regulation. Pharmacol. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura-Kuroda, J.; Komuta, Y.; Kuroda, Y.; Hayashi, M.; Kawano, H. Nicotine-Like Effects of the Neonicotinoid Insecticides Acetamiprid and Imidacloprid on Cerebellar Neurons from Neonatal Rats. PLoS ONE 2012, 7, e32432. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Nishito, Y.; Yanagisawa, H.; Kuroda, Y.; Komuta, Y.; Kawano, H.; Hayashi, M. Neonicotinoid Insecticides Alter the Gene Expression Profile of Neuron-Enriched Cultures from Neonatal Rat Cerebellum. Int. J. Environ. Res. Public Health 2016, 13, 987. [Google Scholar] [CrossRef] [Green Version]
- Christen, V.; Rusconi, M.; Crettaz, P.; Fent, K. Developmental neurotoxicity of different pesticides in PC-12 cells in vitro. Toxicol. Appl. Pharmacol. 2017, 325, 25–36. [Google Scholar] [CrossRef]
- Hirano, T.; Yanai, S.; Takada, T.; Yoneda, N.; Omotehara, T.; Kubota, N.; Minami, K.; Yamamoto, A.; Mantani, Y.; Yokoyama, T.; et al. NOAEL-dose of a neonicotinoid pesticide, clothianidin, acutely induce anxiety-related behavior with human-audible vocalizations in male mice in a novel environment. Toxicol. Lett. 2018, 282, 57–63. [Google Scholar] [CrossRef]
- Nakayama, A.; Yoshida, M.; Kagawa, N.; Nagao, T. The neonicotinoids acetamiprid and imidacloprid impair neurogenesis and alter the microglial profile in the hippocampal dentate gyrus of mouse neonates. J. Appl. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.; Yoon, K.S.; Clark, J.M.; Park, Y. Imidacloprid, a neonicotinoid insecticide, induces insulin resistance. J. Toxicol. Sci. 2013, 38, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şekeroğlu, V.; Şekeroğlu, Z.A.; Demirhan, E. Effects of commercial formulations of deltamethrin and/or thiacloprid on thyroid hormone levels in rat serum. Toxicol. Ind. Health 2014, 30, 40–46. [Google Scholar] [CrossRef]
- Sun, Q.; Xiao, X.; Kim, Y.; Kim, D.; Yoon, K.S.; Clark, J.M.; Park, Y. Imidacloprid Promotes High Fat Diet-Induced Adiposity and Insulin Resistance in Male C57BL/6J Mice. J. Agric. Food Chem. 2016, 64, 9293–9306. [Google Scholar] [CrossRef]
- Sun, Q.; Qi, W.; Xiao, X.; Yang, S.H.; Kim, D.; Yoon, K.S.; Clark, J.M.; Park, Y. Imidacloprid promotes high fat diet-induced adiposity in female C57BL/6J mice and enhance adipogenesis in 3T3-L1 adipocytes via AMPKα-mediated pathway. J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron-Beaudoin, E.; Viau, R.; Sanderson, J.T. Effects of Neonicotinoid Pesticides on Promoter-Specific Aromatase (CYP19) Expression in Hs578t Breast Cancer Cells and the Role of the VEGF Pathway. Environ. Health Perspect. 2018, 126, 047014. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E. Unique and Common Metabolites of Thiamethoxam, Clothianidin, and Dinotefuran in Mice. Chem. Res. Toxicol. 2006, 19, 1549–1556. [Google Scholar] [CrossRef]
- Terayama, H.; Endo, H.; Tsukamoto, H.; Matsumoto, K.; Umezu, M.; Kanazawa, T.; Ito, M.; Sato, T.; Naito, M.; Kawakami, S.; et al. Acetamiprid Accumulates in Different Amounts in Murine Brain Regions. Int. J. Environ. Res. Public Health 2016, 13, 937. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.H.; Tanaka, K.; Sakamoto, H.; Imanaka, M.; Niisoe, T.; Hitomi, T.; Kobayashi, H.; Okuda, H.; Inoue, S.; Kusakawa, K.; et al. Biological Monitoring of Human Exposure to Neonicotinoids Using Urine Samples, and Neonicotinoid Excretion Kinetics. PLoS ONE 2016, 11, e0146335. [Google Scholar] [CrossRef] [Green Version]
- Flores, C.M.; DeCamp, R.M.; Kilo, S.; Rogers, S.W.; Hargreaves, K.M. Neuronal Nicotinic Receptor Expression in Sensory Neurons of the Rat Trigeminal Ganglion: Demonstration of α3β4, a Novel Subtype in the Mammalian Nervous System. J. Neurosci. 1996, 16, 7892–7901. [Google Scholar] [CrossRef] [Green Version]
- Millar, N.S.; Gotti, C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, I.M.D.; Nunes, B.V.F.; Barbosa, D.R.; Pallares, A.M.; Faro, L.R.F. Effects of the neonicotinoids thiametoxam and clothianidin on in vivo dopamine release in rat striatum. Toxicol. Lett. 2010, 192, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Faro, L.; Oliveira, I.; Durán, R.; Alfonso, M. In vivo neurochemical characterization of clothianidin induced striatal dopamine release. Toxicology 2012, 302, 197–202. [Google Scholar] [CrossRef]
- Faro, L.R.; Kim, H.T.; Alfonso, M.; Durán, R. Clothianidin, a neonicotinoid insecticide, activates α4β2, α7 and muscarinic receptors to induce in vivo dopamine release from rat striatum. Toxicology 2019, 152285. [Google Scholar] [CrossRef]
- Nagata, K.; Song, J.H.; Shono, T.; Narahashi, T. Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nitromethylene heterocycle imidacloprid. J. Pharmacol. Exp. Ther. 1998, 285, 731–738. [Google Scholar] [PubMed]
- Khalil, S.R.; Awad, A.; Mohammed, H.H.; Nassan, M.A. Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis in male rats. Environ. Toxicol. Pharmacol. 2017, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, I.; Yamakuni, T. Imidacloprid, a neonicotinoid insecticide, facilitates tyrosine hydroxylase transcription and phenylethanolamine N-methyltransferase mRNA expression to enhance catecholamine synthesis and its nicotine-evoked elevation in PC12D cells. Toxicology 2018, 394, 84–92. [Google Scholar] [CrossRef]
- Benowitz, N.L. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benowitz, N.L.; Gourlay, S.G. Cardiovascular Toxicity of Nicotine: Implications for Nicotine Replacement Therapy. J. Am. Coll. Cardiol. 1997, 29, 1422–1431. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P. Agents Acting at the Neuromuscular Junction and Autonomic Ganglia. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 8th Pergamon ed.; Gilman, A., Rall, T., Nies, A., Taylor, P., Eds.; Pergamon Press: New York, NY, USA, 1990; pp. 166–186. [Google Scholar]
- Marano, G.; Ramirez, A.; Mori, I.; Ferrari, A.U. Sympathectomy inhibits the vasoactive effects of nicotine in conscious rats. Cardiovasc. Res. 1999, 42, 201–205. [Google Scholar] [CrossRef]
- Phua, D.H.; Lin, C.C.; Wu, M.L.; Deng, J.F.; Yang, C.C. Neonicotinoid insecticides: An emerging cause of acute pesticide poisoning. Clin. Toxicol. 2009, 47, 336–341. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, A.; Kumar, A. Accidental human poisoning with a neonicotinoid insecticide, imidacloprid: A rare case report from rural India with a brief review of literature. Egypt. J. Forensic Sci. 2013, 3, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Imamura, T.; Yanagawa, Y.; Nishikawa, K.; Matsumoto, N.; Sakamoto, T. Two cases of acute poisoning with acetamiprid in humans. Clin. Toxicol. 2010, 48, 851–853. [Google Scholar] [CrossRef]
- Dominiak, P.; Fuchs, G.; Toth, S.V.; Grobecker, H. Effects of nicotine and its major metabolites on blood pressure in anaesthetized rats. Klin. Wochenschr. 1985, 63, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Tanus-Santos, J.E.; Sampaio, R.C.; Hyslop, S.; Franchini, K.G.; Moreno, H. Endothelin ETA receptor antagonism attenuates the pressor effects of nicotine in rats. Eur. J. Pharmacol. 2000, 396, 33–37. [Google Scholar] [CrossRef]
- Yamakura, T.; Chavez-Noriega, L.E.; Harris, R.A. Subunit-dependent Inhibition of Human Neuronal Nicotinic Acetylcholine Receptors and Other Ligand-gated Ion Channels by Dissociative Anesthetics Ketamine and Dizocilpine. Anesthesiology 2000, 92, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, M.; Lambov, N.; Penchev, B.; Ivanov, A. Application of Nicotine and Cytisine on the nicotinism treatment. Pharma-News 2003, 32, 18–31. [Google Scholar]
- Hays, J.T.; Ebbert, J.O. Varenicline for Tobacco Dependence. N. Engl. J. Med. 2008, 359, 2018–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selcuk, E.B.; Süngü, M.; Tetik, B.K.; Parlakpınar, H.; Ermiş, N.; Taslidere, E.; Vardı, N.; Yalcinsoy, M.; Sağır, M.; Polat, A.; et al. Evaluation of the cardiovascular effects of varenicline in rats. Drug Des. Dev. Ther. 2015, 9, 5705–5717. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.L.; Spikes, J.J.; Ellis, S. Cardiovascular effects of brevetoxins in dogs. Toxicon 1985, 23, 505–515. [Google Scholar] [CrossRef]
- Taly, A.; Corringer, P.J.; Guedin, D.; Lestage, P.; Changeux, J.P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Alamiddine, Z.; Selvam, B.; Cerón-Carrasco, J.P.; Mathé-Allainmat, M.; Lebreton, J.; Thany, S.H.; Laurent, A.D.; Graton, J.; Questel, J.Y.L. Molecular recognition of thiaclopride by Aplysia californica AChBP: New insights from a computational investigation. J. Comput. Aided Mol. Des. 2015, 29, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Alamiddine, Z.; Selvam, B.; Graton, J.; Laurent, A.D.; Landagaray, E.; Lebreton, J.; Mathé-Allainmat, M.; Thany, S.H.; Questel, J.Y.L. Binding of Sulfoxaflor to Aplysia californica-AChBP: Computational Insights from Multiscale Approaches. J. Chem. Inf. Model. 2019, 59, 3755–3769. [Google Scholar] [CrossRef]
- Taly, A.; Colas, C.; Malliavin, T.; Blondel, A.; Nilges, M.; Corringer, P.J.; Joseph, D. Discrimination of agonists versus antagonists of nicotinic ligands based on docking onto AChBP structures. J. Mol. Graph. Model. 2011, 30, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Mattei, C.; Taly, A.; Soualah, Z.; Saulais, O.; Henrion, D.; Guérineau, N.C.; Verleye, M.; Legros, C. Involvement of the GABAA receptor α subunit in the mode of action of etifoxine. Pharmacol. Res. 2019, 145, 104250. [Google Scholar] [CrossRef]
- Krashia, P.; Moroni, M.; Broadbent, S.; Hofmann, G.; Kracun, S.; Beato, M.; Groot-Kormelink, P.J.; Sivilotti, L.G. Human α3β4 Neuronal Nicotinic Receptors Show Different Stoichiometry if They Are Expressed in Xenopus Oocytes or Mammalian HEK293 Cells. PLoS ONE 2010, 5, e13611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespin, L.; Legros, C.; List, O.; Tricoire-Leignel, H.; Mattei, C. Injection of insect membrane in Xenopus oocyte: An original method for the pharmacological characterization of neonicotinoid insecticides. J. Pharmacol. Toxicol. Methods 2016, 77, 10–16. [Google Scholar] [CrossRef]
- Blackburn, M.B.; Andrade, M.A.; Toney, G.M. Hypothalamic PVN contributes to acute intermittent hypoxia-induced sympathetic but not phrenic long-term facilitation. J. Appl. Physiol. 2018, 124, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Haniuda, M.; Itoh, N.; Chiba, S. Time-dependent enhancement of xylazine-induced, alpha-2 adrenoceptor-mediated vasoconstriction in isolated and perfused canine pulmonary veins. J. Pharmacol. Exp. Ther. 1989, 249, 340–347. [Google Scholar]
- Wang, Z.; Brooks, B.W.; Zeng, E.Y.; You, J. Comparative mammalian hazards of neonicotinoid insecticides among exposure durations. Environ. Int. 2019, 125, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-coffee: A novel method for fast and accurate multiple sequence alignment11Edited by J. Thornton. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [Green Version]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons Learned in Empirical Scoring with smina from the CSAR 2011 Benchmarking Exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Lehmler, H.J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Taly, A.; Bourreau, J.; De Nardi, F.; Legendre, C.; Henrion, D.; Guérineau, N.C.; Legros, C.; Mattei, C.; Tricoire-Leignel, H. Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure. Int. J. Mol. Sci. 2021, 22, 5106. https://doi.org/10.3390/ijms22105106
Park J, Taly A, Bourreau J, De Nardi F, Legendre C, Henrion D, Guérineau NC, Legros C, Mattei C, Tricoire-Leignel H. Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure. International Journal of Molecular Sciences. 2021; 22(10):5106. https://doi.org/10.3390/ijms22105106
Chicago/Turabian StylePark, Joohee, Antoine Taly, Jennifer Bourreau, Frédéric De Nardi, Claire Legendre, Daniel Henrion, Nathalie C. Guérineau, Christian Legros, César Mattei, and Hélène Tricoire-Leignel. 2021. "Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure" International Journal of Molecular Sciences 22, no. 10: 5106. https://doi.org/10.3390/ijms22105106
APA StylePark, J., Taly, A., Bourreau, J., De Nardi, F., Legendre, C., Henrion, D., Guérineau, N. C., Legros, C., Mattei, C., & Tricoire-Leignel, H. (2021). Partial Agonist Activity of Neonicotinoids on Rat Nicotinic Receptors: Consequences over Epinephrine Secretion and In Vivo Blood Pressure. International Journal of Molecular Sciences, 22(10), 5106. https://doi.org/10.3390/ijms22105106