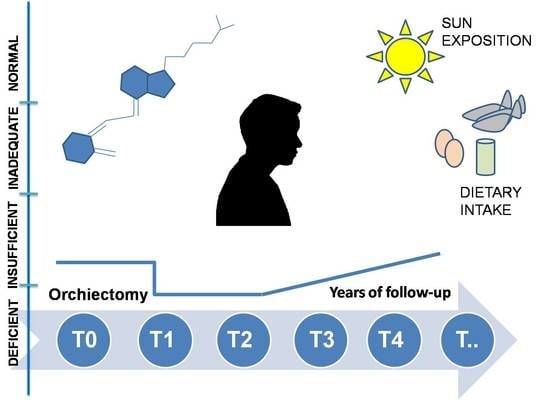
Vitamin D Deficiency in Testicular Cancer Survivors: A Systematic Review
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scott, A.R.; Stoltzfus, K.C.; Tchelebi, L.T.; Trifiletti, D.M.; Lehrer, E.J.; Rao, P.; Bleyer, A.; Zaorsky, N.G. Trends in cancer incidence in US adolescents and young adults, 1973–2015. JAMA Netw. Open 2020, 3, e2027738. [Google Scholar] [CrossRef]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H. Testicular cancer. Nat. Rev. Dis. Prim. 2018, 4, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Dinh, P.; Ardeshir-Rouhani-Fard, S.; Schaffer, K.; Fossa, S.D.; Travis, L.B. Toxicities associated with cisplatin-based chemotherapy and radiotherapy in long-term testicular cancer survivors. Adv. Urol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wehr, E.; Pilz, S.; Boehm, B.O.; März, W.; Obermayer-Pietsch, B. Association of vitamin D status with serum androgen levels in men. Clin. Endocrinol. 2010, 73, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Weinstein, S.J.; Layne, T.M.; Albanes, D. Vitamin D and cancer risk and mortality: State of the science, gaps, and challenges. Epidemiol. Rev. 2017, 39, 28–48. [Google Scholar] [CrossRef]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Charlton, K.; Thakur, S.; Ribeiro, H.; Lanham-New, S.A. Future perspectives in addressing the global issue of vitamin D deficiency. Proc. Nutr. Soc. 2020, 79, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef]
- O’Connor, A.; Benelam, B. An update on UK Vitamin D intakes and status, and issues for food fortification and supplementation. Nutr. Bull. 2011, 36, 390–396. [Google Scholar] [CrossRef]
- Margulies, S.L.; Kurian, D.; Elliott, M.S.; Han, Z. Vitamin D deficiency in patients with intestinal malabsorption syndrome—Think in and outside the gut. J. Dig. Dis. 2015, 16, 617–633. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [Green Version]
- Norman, A.W.; Okamura, W.H.; Bishop, J.E.; Henry, H.L. Update on biological actions of 1α,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D. Mol. Cell Endocrinol. 2002, 197, 1–13. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New aspects of vitamin D metabolism and action—Addressing the skin as source and target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. 2008, 88, 491S–499S. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Sadeghnia, H.R.; Tabatabaeizadeh, S.A.; Bahrami-Taghanaki, H.; Behboodi, N.; Esmaeili, H.; Ferns, G.A.; Mobarhan, M.G.; Avan, A. Genetic and epigenetic factors influencing vitamin D status. J. Cell Physiol. 2018, 233, 4033–4043. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Li, S.; Cruz, J.D.L.; Bikle, D.D. New developments in our understanding of vitamin metabolism, action and treatment. Metabolism 2019, 98, 112–120. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468.e3. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D signaling in the context of innate immunity: Focus on human monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Essen, M.R.; Kongsbak, M.; Schjerling, P.; Olgaard, K.; Ødum, N.; Geisler, C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010, 11, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, A.M.; Wang, E.; Chouchane, A.I. Vitamin D and the pathophysiology of inflammatory skin diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Gunta, S.S.; Thadhani, R.I.; Mak, R.H. The effect of vitamin D status on risk factors for cardiovascular disease. Nat. Rev. Nephrol. 2013, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B. Vitamin D and male reproduction. Nat. Rev. Endocrinol. 2014, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, G.; Sudano, M.; Salvio, G.; Cutini, M.; Muscogiuri, G.; Corona, G.; Balercia, G. Vitamin D and male sexual function: A transversal and longitudinal study. Int. J. Endocrinol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo, S. Novel roles of 1α,25(OH)2D3 on DNA repair provide new strategies for breast cancer treatment. J. Steroid Biochem. Mol. Biol. 2014, 144, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Dovnik, A.; Dovnik, N.F. Vitamin D and ovarian cancer: Systematic review of the literature with a focus on molecular mechanisms. Cells 2020, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Cheng, X.; Wang, W.; Zhang, J. Vitamin D regulates cell viability, migration and proliferation by suppressing galectin-3 (Gal-3) gene in ovarian cancer cells. J. Biosci. 2020, 45, 1–10. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Bandera Merchan, B.; Morcillo, S.; Martin-Nuñez, G.; Tinahones, F.J.; Macías-González, M. The role of vitamin D and VDR in carcinogenesis: Through epidemiology and basic sciences. J. Steroid Biochem. Mol. Biol. 2017, 167, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.-S.; Dixon-Suen, S.C.; Han, X.; An, J.; Esophageal Cancer Consortium; 23 and Me Research Team; Liyanage, U.; Dusingize, J.C.; Schumacher, J.; Gockel, I.; et al. A comprehensive re-assessment of the association between vitamin D and cancer susceptibility using Mendelian randomization. Nat. Commun. 2021, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Lee, D.H.; Greenwood, D.C.; Manson, J.E.; Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: A meta-analysis of randomized controlled trials. Ann. Oncol. 2019, 30, 733–743. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the VITAL randomized clinical trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Foresta, C.; Selice, R.; Mambro, A.D.; Strapazzon, G. Testiculopathy and vitamin D insufficiency. Lancet 2010, 376, 1301. [Google Scholar] [CrossRef]
- Foresta, C.; Selice, R.; Toni, L.D.; De Toni, L.; Di Mambro, A.; Carraro, U.; Plebani, M.; Garolla, A. Altered bone status in unilateral testicular cancer survivors: Role of CYP2R1 and its luteinizing hormone-dependency. J. Endocrinol. Investig. 2013, 36, 379–384. [Google Scholar] [CrossRef]
- Willemse, P.M.; Hamdy, N.A.T.; Kam, M.L.D.; Burggraaf, J.; Osanto, S. Changes in bone mineral density in newly diagnosed testicular cancer patients after anticancer treatment. J. Clin. Endocrinol. Metab. 2014, 99, 4101–4108. [Google Scholar] [CrossRef] [Green Version]
- Schepisi, G.; Padova, S.D.; Scarpi, E.; Lolli, C.; Gurioli, G.; Menna, C.; Burgio, S.L.; Rossi, L.; Gallà, V.; Casadio, V.; et al. Vitamin D status among long-term survivors of testicular cancer. Oncotarget 2017, 8, 36780–36786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghezzi, M.; Toni, L.D.; Palego, P.; Menegazzo, M.; Faggian, E.; Berretta, M.; Fiorica, F.; De Rocco Ponce, M.; Foresta, C.; Garolla, A. Increased risk of testis failure in testicular germ cell tumor survivors undergoing radiotherapy. Oncotarget 2018, 9, 3060–3068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nappi, L.; Ottaviano, M.; Rescigno, P.; Fazli, L.; Gleave, M.E.; Damiano, V.; De Placido, S.; Palmieri, G. Long term deficiency of vitamin D in germ cell testicular cancer survivors. Oncotarget 2018, 9, 21078–21085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieckmann, K.-P.; Andura, O.; Pichlmeier, U.; Otte, K.M.; Isbarn, H.; Wülfing, C. Does orchiectomy cause decrease of vitamin D serum levels in patients with testicular tumors? Basic Clin. Androl. 2021, in press. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, H.M.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.M.; Tajar, A.; Pye, S.R.; Boonen, S.; Vanderschueren, D.; Bouillon, R.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Finn, J.D.; et al. Association of hypogonadism with vitamin D status: The european male ageing study. Eur. J. Endocrinol. 2012, 166, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Nimptsch, K.; Platz, E.A.; Willett, W.C.; Giovannucci, E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin. Endocrinol. 2012, 77, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Foresta, C.; Strapazzon, G.; Toni, L.D.; De Toni, L.; Perilli, L.; Di Mambro, A.; Muciaccia, B.; Sartori, L.; Selice, R. Bone mineral density and testicular failure: Evidence for a role of vitamin D 25-hydroxylase in human testis. J. Clin. Endocrinol. Metab. 2011, 96, E646–E652. [Google Scholar] [CrossRef] [Green Version]
- Robien, K.; Strayer, L.G.; Majhail, N.; Lazovich, D.; Baker, K.S.; Smith, A.R.; Mulrooney, D.A.; Burns, L.J. Vitamin D status among long-term survivors of hematopoietic cell transplantation. Bone Marrow Transplant. 2011, 46, 1472–1479. [Google Scholar] [CrossRef] [Green Version]
- De Giorgi, U.; Demirer, T.; Wandt, H.; Taverna, C.; Siegert, W.; Bornhauser, M.; Kozak, T.; Papiani, G.; Ballardini, M.; Rosti, G. Solid Tumor Working Party of the European Group for Blood and Marrow Transplantation. Second-line high-dose chemotherapy in patients with mediastinal and retroperitoneal primary non-seminomatous germ cell tumors: the EBMT experience. Ann. Oncol. 2005, 16, 146–151. [Google Scholar]
- De Giorgi, U.; Rosti, G.; Slavin, S.; Yaniv, I.; Harousseau, J.L.; Ladenstein, R.; Demirer, T.; Dini, G.; Pizzocaro, G. European Group for Blood and Marrow Transplantation Solid Tumours and Paediatric Disease Working Parties. Salvage high-dose chemotherapy for children with extragonadal germ-cell tumours. Br. J. Cancer. 2005, 93, 412–417. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Preziosi, P.; Maamer, M.; Arnaud, S.; Galan, P.; Hercberg, S.; Meunier, P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997, 7, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. The estimated benefits of vitamin D for Germany. Mol. Nutr. Food Res. 2010, 54, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.; Khabarova, Y.; Vershubsky, G.; Ateeva, Y.; Ryzhaenkov, V. Vitamin D status of northern indigenous people of Russia leading traditional and “modernized” way of life. Int. J. Circumpolar Health 2014, 73, 26038. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, J.; Ståhl, O.; Cwikiel, M.; Cavallin-Ståhl, E.; Giwercman, Y.; Salmonson, E.C.; Giwercman, A. Risk factors for post-treatment hypogonadism in testicular cancer patients. Eur. J. Endocrinol. 2008, 158, 561–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondrusova, M.; Ondrus, D.; Dusek, L.; Spanikova, B. Damage of hormonal function and bone metabolism in long-term survivors of testicular cancer. Neoplasma 2009, 56, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Ondrusova, M.; Spanikova, B.; Sevcikova, K.; Ondrus, D. Testosterone deficiency and bone metabolism damage in testicular cancer survivors. Am. J. Mens Health 2018, 12, 628–633. [Google Scholar] [CrossRef]
- Sprauten, M.; Brydøy, M.; Haugnes, H.S.; Cvancarova, M.; Bjøro, T.; Bjerner, J.; Fosså, S.D.; Oldenburg, J. Longitudinal serum testosterone, luteinizing hormone, and follicle-stimulating hormone levels in a population-based sample of long-term testicular cancer survivors. J. Clin. Oncol. 2014, 32, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1a-hydroxylasein human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Huddart, R.A.; Norman, A.; Moynihan, C.; Horwich, A.; Parker, C.; Nicholls, E.; Dearnaley, D.P. Fertility, gonadal and sexual function in survivors of testicular cancer. Br. J. Cancer 2005, 93, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anagnostis, P.; Karras, S.; Goulis, D.G. Vitamin D in human reproduction: A narrative review. Int. J. Clin. Pract. 2013, 67, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.T.; Hill, O.; Nangia, A.K. Vitamin D receptor found in human sperm. Urology 2006, 68, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Cito, G.; Cocci, A.; Micelli, E.; Gabutti, A.; Russo, G.I.; Coccia, M.E.; Franco, G.; Serni, S.; Carini, M.; Natali, A. Vitamin D and male fertility: An updated review. World J. Mens Health 2020, 38, 164–177. [Google Scholar] [CrossRef]
- Angelis, C.D.; Galdiero, M.; Pivonello, C.; Garifalos, F.; Menafra, D.; Cariati, F.; Salzano, C.; Galdiero, G.; Piscopo, M.; Vece, A.; et al. The role of vitamin D in male fertility: A focus on the testis. Rev. Endocr. Metab. Disord. 2017, 18, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Nielsen, J.E.; Jørgensen, A.; Rajpert-De Meyts, E.; Kristensen, D.M.; Jørgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremmer, F.; Thelen, P.; Pottek, T.; Behnes, C.; Radzun, H.; Schweyer, S. Expression and function of the vitamin D receptor in malignant germ cell tumour of the testis. Anticancer Res. 2012, 32, 341–349. [Google Scholar] [PubMed]
- Baker, K.S.; Broderick, G.; Day, A. NCCN Guidelines Version 1.2021 Survivorship NCCN Guidelines Panel Disclosures. Available online: www.nccn.org/patients (accessed on 12 April 2021).
- Gilligan, T.; Lin, D.W.; Aggarwal, R.; Chism, D.; Cost, N.; Derweesh, I.H.; Emamekhoo, H.; Feldman, D.R.; Geynisman, D.M.; Hancock, S.L.; et al. NCCN Clinical Practice Guidelines in Oncology. Testicular Cancer. Version 1.2021—Nov. 5. 2020. Available online: www.nccn.org/guidelines (accessed on 12 April 2021).
- Honecker, F.; Aparicio, J.; Berney, D.; Beyer, J.; Bokemeyer, C.; Cathomas, R.; Clarke, N.; Cohn-Cedermark, G.; Daugaard, G.; Dieckmann, K.P.; et al. ESMO consensus conference on testicular germ cell cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, 1658–1686. [Google Scholar] [CrossRef]
- Laguna, M.P.; Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Boormans, J.; Cohn-Cedermark, G.; Fizazi, K.; Gremmels, H.; Horwich, A.; et al. Testicular Cancer EAU Guidelines on. Available online: www.uroweb.org/guideline/testicular-cancer/ (accessed on 12 April 2021).
- Anderson, J.L.; Vanwoerkom, R.C.; Horne, B.D.; Bair, T.L.; May, H.T.; Lappé, D.L.; Muhlestein, J.B. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am. Heart J. 2011, 162, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Meredith, A.J.; McManus, B.M. Vitamin D in heart failure. J. Card. Fail. 2013, 19, 692–711. [Google Scholar] [CrossRef]
- Willemse, P.M.; Hamdy, N.A.T.; Van Wulften, L.; van Steijn-van Tol, A.Q.; Putter, H.; Osanto, S. Prevalence of vertebral fractures independent of BMD and anticancer treatment in patients with testicular germ cell tumors. J. Clin. Endocrinol. Metab. 2010, 95, 4933–4942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]

| Study and Country | Patients | Hypo-Vit D Cut-Off Level | Hypo-Vit D in TC Survivors (%) | Hypo-Vit D in Healthy Control Group (%) | Time of Sample Collection |
|---|---|---|---|---|---|
| Foresta 2010 [39] ITA | 15 | <50 nmol/L | 60% | NA | 3–5 years |
| Foresta 2013 [40] ITA | 125 | <50 nmol/L | 73.6% | 7.3% | At baseline and at 3 months |
| Willemse 2014 [41] NED | 63 | <50 nmol/L | 36.5% | NR | At baseline and then annually for 5 years |
| Schepisi 2017 [42] ITA | 61 | <75 nmol/L <50 nmol/L <25 nmol/L | 81 | 0% | ≥3 years |
| Ghezzi 2018 [43] ITA | 192 | <50 nmol/L | Survivors RT (T0) 27.9% 21.8% (T1) 32.8% 59.9% (T2) 47.6% 83.8% | NA | At baseline and then annually for 2 years |
| Nappi 2018 [44] ITA | 82 | <75 nmol/L <50 nmol/L <25 nmol/L | (T1) 85% (T2) 66% (T3) 80% (T4) 72% (T5) 81% | NA | At baseline, every 3 months for the first 2 years, then every six months until the fifth year |
| Dieckmann 2021 [45] DEU | 177 | <75 nmol/L <50 nmol/L <25 nmol/L | (Tpre-s) 78% (Tpos-s) 82% (T1) 97% (T2) 91% (T3) 77% | Cohort 2 79.8% Cohort 3 78% | Before and immediately after surgery, and then at 5 other time-points until 2 years of follow-up |
| Study | 25-OH VitaminD | Calcium | Phosphorus | PTH | Calcitonin | FSH | LH | Testosterone | Beta-Estradiol | Progesterone |
|---|---|---|---|---|---|---|---|---|---|---|
| Foresta 2010 [39] | p < 0.0001 | NA | NA | NA | NA | NR | supplemented | NA | NA | |
| Foresta 2013 [40] | p < 0.00001 | p < 0.00001 | NA | p < 0.00001 | p < 0.00001 | NA | ||||
| Willemse 2014 [41] | (p = NR) | NR | NR | NR | NA | NA | ||||
| Schepisi 2017 [42] | p = 0.047 | p = 0.002 | ns | p = 0.996 | ns | |||||
| Ghezzi 2018 [43] | p= 0.421 | NA | p = 0.174 | NA | NA | |||||
| Nappi 2018 [44] | (p = NR) | NA | NA | NA | NA | (p = NR) | NA | NA | ||
| Dieckmann 2021 [45] | (p = 0.161) | NA | NA | NA | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepisi, G.; Gianni, C.; Bleve, S.; De Padova, S.; Menna, C.; Lolli, C.; Filograna, A.; Conteduca, V.; Urbini, M.; Gallà, V.; et al. Vitamin D Deficiency in Testicular Cancer Survivors: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 5145. https://doi.org/10.3390/ijms22105145
Schepisi G, Gianni C, Bleve S, De Padova S, Menna C, Lolli C, Filograna A, Conteduca V, Urbini M, Gallà V, et al. Vitamin D Deficiency in Testicular Cancer Survivors: A Systematic Review. International Journal of Molecular Sciences. 2021; 22(10):5145. https://doi.org/10.3390/ijms22105145
Chicago/Turabian StyleSchepisi, Giuseppe, Caterina Gianni, Sara Bleve, Silvia De Padova, Cecilia Menna, Cristian Lolli, Alessia Filograna, Vincenza Conteduca, Milena Urbini, Valentina Gallà, and et al. 2021. "Vitamin D Deficiency in Testicular Cancer Survivors: A Systematic Review" International Journal of Molecular Sciences 22, no. 10: 5145. https://doi.org/10.3390/ijms22105145
APA StyleSchepisi, G., Gianni, C., Bleve, S., De Padova, S., Menna, C., Lolli, C., Filograna, A., Conteduca, V., Urbini, M., Gallà, V., Casadei, C., Rosti, G., & De Giorgi, U. (2021). Vitamin D Deficiency in Testicular Cancer Survivors: A Systematic Review. International Journal of Molecular Sciences, 22(10), 5145. https://doi.org/10.3390/ijms22105145