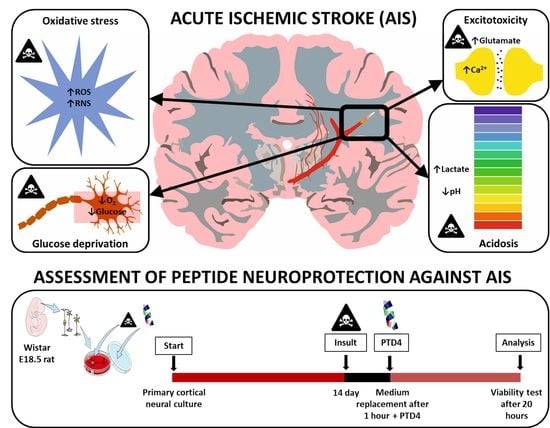
PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke
Abstract
:1. Introduction
2. Results
2.1. Ala-Substitution Favors Helicity of PTD4 Compared to Tat(49–57)-NH2
2.2. Conformational Structure of PTD4 Predisposes It to Enter the Cell Interior
2.3. PTD4 Adopts the Favorable Structure in the Membrane-Mimicking Environment
2.4. PTD4 Expresses no or Minor Neurotoxicity in Primary Neural Cortical Cultures
2.5. PTD4 Is Pro-Viable in an In Vitro Model of Acute Ischemic Stroke
3. Discussion
4. Materials and Methods
4.1. Peptides Synthesis and Purification
4.2. Circular Dichroism Spectroscopy
4.3. Molecular Dynamics Calculations
4.4. Primary Neural Cortical Cultures
4.5. Optimization of an In Vitro Model of Acute Ischemic Stroke
4.5.1. Optimization of Glucose Deprivation
4.5.2. Optimization of the Sodium Azide-Induced Inhibition of Cellular Respiration
4.5.3. Optimization of Acidosis
4.5.4. Optimization of Excitotoxicity Models
4.6. Treatment of Neural Cultures with Peptides
4.7. Pro-Viable Effect of the Peptides in an In Vitro Model of Acute Ischemic Stroke
4.8. Neural Viability Assessments
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Acute ischemic stroke |
| ARM | Arginine rich motif |
| BBB | Blood–brain barrier |
| CD | Circular dichroism |
| CPP | Cell penetrating peptide |
| CTB | CellTiter-Blue |
| FDA | Food and drug administration |
| HAND | HIV-associated neurological disorder |
| HIV | Human immunodeficiency virus |
| MD | Molecular dynamics |
| NMDA | N-methyl D-aspartate |
| NMDAR | NMDA receptor |
| NMDAR-PSD | NMDAR postsynaptic density protein |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| OS | Oxidative stress |
| PTD | Protein transduction domain |
| PTM | Post-translational modification |
| RRP | Arginine rich peptide |
| rtPA | Recombinant tissue plasminogen activator |
| TAT | Transactivator of transcription |
| TFE | Trifluoroethanol |
References
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Luengo-Fernandez, R.; Violato, M.; Candio, P.; Leal, J. Economic burden of stroke across Europe: A population-based cost analysis. Eur. Stroke J. 2020, 5, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef]
- Meloni, B.P.; Brookes, L.M.; Clark, V.W.; Cross, J.L.; Edwards, A.B.; Anderton, R.S.; Hopkins, R.M.; Hoffmann, K.; Knuckey, N.W. Poly-arginine and arginine-rich peptides are neuroprotective in stroke models. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2015, 35, 993–1004. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Ezzet, K.A. Reversal of acute ischemic stroke after THA using tissue plasminogen activator. Orthopedics 2013, 36, e676–e678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kardani, K.; Milani, A.; H. Shabani, S.; Bolhassani, A. Cell penetrating peptides: The potent multi-cargo intracellular carriers. Expert Opin. Drug Deliv. 2019, 16, 1227–1258. [Google Scholar] [CrossRef]
- Rusiecka, I.; Ruczynski, J.; Kozlowska, A.; Backtrog, E.; Mucha, P.; Kocic, I.; Rekowski, P. TP10-Dopamine Conjugate as a Potential Therapeutic Agent in the Treatment of Parkinson’s Disease. Bioconjug. Chem. 2019, 30, 760–774. [Google Scholar] [CrossRef]
- Mishra, A.; Lai, G.H.; Schmidt, N.W.; Sun, V.Z.; Rodriguez, A.R.; Tong, R.; Tang, L.; Cheng, J.; Deming, T.J.; Kamei, D.T.; et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 16883–16888. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N.; Mishra, A.; Lai, G.H.; Wong, G.C. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010, 584, 1806–1813. [Google Scholar] [CrossRef] [Green Version]
- Fineberg, K.; Fineberg, T.; Graessmann, A.; Luedtke, N.W.; Tor, Y.; Lixin, R.; Jans, D.A.; Loyter, A. Inhibition of nuclear import mediated by the Rev-arginine rich motif by RNA molecules. Biochemistry 2003, 42, 2625–2633. [Google Scholar] [CrossRef] [PubMed]
- Oller-Salvia, B.; Sanchez-Navarro, M.; Giralt, E.; Teixido, M. Blood-brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarantseva, S.V.; Bol’shakova, O.I.; Timoshenko, S.I.; Kolobov, A.A.; Vitek, M.P.; Shvartsman, A.L. Protein transduction domain peptide mediates delivery to the brain via the blood-brain barrier in Drosophila. Biomeditsinskaia Khimiia 2009, 55, 41–49. [Google Scholar] [CrossRef]
- Kamori, D.; Ueno, T. HIV-1 Tat and Viral Latency: What We Can Learn from Naturally Occurring Sequence Variations. Front. Microbiol. 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meloni, B.P.; Milani, D.; Edwards, A.B.; Anderton, R.S.; O′Hare Doig, R.L.; Fitzgerald, M.; Palmer, T.N.; Knuckey, N.W. Neuroprotective peptides fused to arginine-rich cell penetrating peptides: Neuroprotective mechanism likely mediated by peptide endocytic properties. Pharmacol. Ther. 2015, 153, 36–54. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Alvarado, M.; Blanco, F.J.; Niemann, H.; Serrano, L. Role of beta-turn residues in beta-hairpin formation and stability in designed peptides. J. Mol. Biol. 1997, 273, 898–912. [Google Scholar] [CrossRef]
- Reiersen, H.; Rees, A.R. Trifluoroethanol may form a solvent matrix for assisted hydrophobic interactions between peptide side chains. Protein Eng. 2000, 13, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Vives, E. Cellular uptake [correction of utake] of the Tat peptide: An endocytosis mechanism following ionic interactions. J. Mol. Recognit. JMR 2003, 16, 265–271. [Google Scholar] [CrossRef]
- Ho, A.; Schwarze, S.R.; Mermelstein, S.J.; Waksman, G.; Dowdy, S.F. Synthetic protein transduction domains: Enhanced transduction potential in vitro and in vivo. Cancer Res. 2001, 61, 474–477. [Google Scholar]
- Zou, L.L.; Ma, J.L.; Wang, T.; Yang, T.B.; Liu, C.B. Cell-penetrating Peptide-mediated therapeutic molecule delivery into the central nervous system. Curr. Neuropharmacol. 2013, 11, 197–208. [Google Scholar] [CrossRef]
- Meloni, B.P.; Craig, A.J.; Milech, N.; Hopkins, R.M.; Watt, P.M.; Knuckey, N.W. The neuroprotective efficacy of cell-penetrating peptides TAT, penetratin, Arg-9, and Pep-1 in glutamic acid, kainic acid, and in vitro ischemia injury models using primary cortical neuronal cultures. Cell. Mol. Neurobiol. 2014, 34, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, A.K.; Buchillier, V.; Mathieu, M.; Chen, J.; Ortis, F.; Ladriere, L.; Allaman-Pillet, N.; Poirot, O.; Kellenberger, S.; Beckmann, J.S.; et al. Cell-permeable peptides induce dose- and length-dependent cytotoxic effects. Biochim. Biophys. Acta 2007, 1768, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.A.; Dumaop, W.; Crews, L.; Adame, A.; Spencer, B.; Metcalf, J.; He, J.; Rockenstein, E.; Masliah, E. Mechanisms of HIV-1 Tat neurotoxicity via CDK5 translocation and hyper-activation: Role in HIV-associated neurocognitive disorders. Curr. HIV Res. 2015, 13, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagashev, A.; Sawaya, B.E. Roles and functions of HIV-1 Tat protein in the CNS: An overview. Virol. J. 2013, 10, 358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, Q.; Yao, G.; Zhang, G.Y.; Zhang, J.; Wang, J.; Zhao, P.; Liu, J. Disruption of GluR2/GAPDH Complex Interaction by TAT-GluR2NT1-3-2 Peptide Protects against Neuronal Death Induced by Epilepsy. Ann. Clin. Lab. Sci. 2018, 48, 460–468. [Google Scholar]
- Rizzuti, M.; Nizzardo, M.; Zanetta, C.; Ramirez, A.; Corti, S. Therapeutic applications of the cell-penetrating HIV-1 Tat peptide. Drug Discov. Today 2015, 20, 76–85. [Google Scholar] [CrossRef]
- Meloni, B.P.; Milani, D.; Cross, J.L.; Clark, V.W.; Edwards, A.B.; Anderton, R.S.; Blacker, D.J.; Knuckey, N.W. Assessment of the Neuroprotective Effects of Arginine-Rich Protamine Peptides, Poly-Arginine Peptides (R12-Cyclic, R22) and Arginine-Tryptophan-Containing Peptides Following In Vitro Excitotoxicity and/or Permanent Middle Cerebral Artery Occlusion in Rats. Neuromolecular Med. 2017, 19, 271–285. [Google Scholar] [CrossRef]
- Peng, J.; Rao, Y.; Yang, X.; Jia, J.; Wu, Y.; Lu, J.; Tao, Y.; Tu, W. Targeting neuronal nitric oxide synthase by a cell penetrating peptide Tat-LK15/siRNA bioconjugate. Neurosci. Lett. 2017, 650, 153–160. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Vitiello, M.; Morelli, G.; Galdiero, M. Peptide-lipid interactions: Experiments and applications. Int. J. Mol. Sci. 2013, 14, 18758–18789. [Google Scholar] [CrossRef] [Green Version]
- MacDougall, G.; Anderton, R.S.; Edwards, A.B.; Knuckey, N.W.; Meloni, B.P. The Neuroprotective Peptide Poly-Arginine-12 (R12) Reduces Cell Surface Levels of NMDA NR2B Receptor Subunit in Cortical Neurons; Investigation into the Involvement of Endocytic Mechanisms. J. Mol. Neurosci. 2017, 61, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Holloway, P.M.; Gavins, F.N. Modeling Ischemic Stroke In Vitro: Status Quo and Future Perspectives. Stroke 2016, 47, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Ahmad, A.; Taboada, C.B.; Gassmann, M.; Ogunshola, O.O. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011, 31, 693–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilling, T.; Korte, D.; Hoheisel, D.; Galla, H.J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. 1998, 71, 1151–1157. [Google Scholar] [CrossRef]
- Zhang, W.; Smith, C.; Howlett, C.; Stanimirovic, D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J. Cereb. Blood Flow Metab. 2000, 20, 967–978. [Google Scholar] [CrossRef] [Green Version]
- Haile, Y.; Fu, W.; Shi, B.; Westaway, D.; Baker, G.; Jhamandas, J.; Giuliani, F. Characterization of the NT2-derived neuronal and astrocytic cell lines as alternative in vitro models for primary human neurons and astrocytes. J. Neurosci. Res. 2014, 92, 1187–1198. [Google Scholar] [CrossRef]
- Selvaraj, V.; Jiang, P.; Chechneva, O.; Lo, U.G.; Deng, W. Differentiating human stem cells into neurons and glial cells for neural repair. Front Biosci. 2012, 17, 65–89. [Google Scholar] [CrossRef] [Green Version]
- Durnaoglu, S.; Genc, S.; Genc, K. Patient-specific pluripotent stem cells in neurological diseases. Stem Cells Int. 2011, 2011, 212487. [Google Scholar] [CrossRef] [Green Version]
- Amemori, T.; Romanyuk, N.; Jendelova, P.; Herynek, V.; Turnovcova, K.; Prochazka, P.; Kapcalova, M.; Cocks, G.; Price, J.; Sykova, E. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Res. Ther. 2013, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Peretz, H.; Talpalar, A.E.; Vago, R.; Baranes, D. Superior survival and durability of neurons and astrocytes on 3-dimensional aragonite biomatrices. Tissue Eng. 2007, 13, 461–472. [Google Scholar] [CrossRef]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Antonic, A.; Sena, E.S.; Donnan, G.A.; Howells, D.W. Human in vitro models of ischaemic stroke: A test bed for translation. Transl. Stroke Res. 2012, 3, 306–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalafatovic, D.; Giralt, E. Cell-Penetrating Peptides: Design Strategies beyond Primary Structure and Amphipathicity. Molecules 2017, 22, 1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyk, A.; Mucha, P.; Rekowski, P.; Giel-Pietraszuk, M.; Barciszewski, J. Synthesis and circular dichroism studies of HIV-1 Tat arginine rich domain analogues substituted in Arg 52 position. Pol. J. Chem. 1999, 73, 879–883. [Google Scholar]
- Ruzza, P.; Biondi, B.; Marchiani, A.; Antolini, N.; Calderan, A. Cell-Penetrating Peptides: A Comparative Study on Lipid Affinity and Cargo Delivery Properties. Pharmaceuticals 2010, 3, 1045–1062. [Google Scholar] [CrossRef] [Green Version]
- Ruzza, P.; Calderan, A.; Guiotto, A.; Osler, A.; Borin, G. Tat cell-penetrating peptide has the characteristics of a poly(proline) II helix in aqueous solution and in SDS micelles. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2004, 10, 423–426. [Google Scholar] [CrossRef]
- Lam, S.L.; Hsu, V.L. NMR identification of left-handed polyproline type II helices. Biopolymers 2003, 69, 270–281. [Google Scholar] [CrossRef]
- Roccatano, D.; Colombo, G.; Fioroni, M.; Mark, A.E. Mechanism by which 2,2,2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: A molecular dynamics study. Proc. Natl. Acad. Sci. USA 2002, 99, 12179–12184. [Google Scholar] [CrossRef] [Green Version]
- Diaz, M.D.; Fioroni, M.; Burger, K.; Berger, S. Evidence of complete hydrophobic coating of bombesin by trifluoroethanol in aqueous solution: An NMR spectroscopic and molecular dynamics study. Chemistry 2002, 8, 1663–1669. [Google Scholar] [CrossRef]
- Fort, A.G.; Spray, D.C. Trifluoroethanol reveals helical propensity at analogous positions in cytoplasmic domains of three connexins. Biopolymers 2009, 92, 173–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, P.; Baldwin, R.L. Mechanism of helix induction by trifluoroethanol: A framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry 1997, 36, 8413–8421. [Google Scholar] [CrossRef]
- Krimm, S.; Mark, J.E. Conformations of polypeptides with ionized side chains of equal length. Proc. Natl. Acad. Sci. USA 1968, 60, 1122–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzwarth, G.; Doty, P. The Ultraviolet Circular Dichroism of Polypeptides. J. Am. Chem. Soc. 1965, 87, 218–228. [Google Scholar] [CrossRef]
- Edwards, A.B.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. Characterisation of neuroprotective efficacy of modified poly-arginine-9 (R9) peptides using a neuronal glutamic acid excitotoxicity model. Mol. Cell. Biochem. 2017, 426, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Post-translational Modifications of Natural Antimicrobial Peptides and Strategies for Peptide Engineering. Curr. Biotechnol. 2012, 1, 72–79. [Google Scholar] [CrossRef]
- Yamada, T.; Signorelli, S.; Cannistraro, S.; Beattie, C.W.; Bizzarri, A.R. Chirality switching within an anionic cell-penetrating peptide inhibits translocation without affecting preferential entry. Mol. Pharm. 2015, 12, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Pujals, S.; Sabido, E.; Tarrago, T.; Giralt, E. all-D proline-rich cell-penetrating peptides: A preliminary in vivo internalization study. Biochem. Soc. Trans. 2007, 35, 794–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdurmen, W.P.; Bovee-Geurts, P.H.; Wadhwani, P.; Ulrich, A.S.; Hallbrink, M.; van Kuppevelt, T.H.; Brock, R. Preferential uptake of L- versus D-amino acid cell-penetrating peptides in a cell type-dependent manner. Chem. Biol. 2011, 18, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Gong, C.; Ma, Y.; Fan, F.; Luo, M.; Yang, F.; Zhang, Y.H. Direct cytosolic delivery of cargoes in vivo by a chimera consisting of D- and L-arginine residues. J. Control. Release Off. J. Control. Release Soc. 2012, 162, 286–294. [Google Scholar] [CrossRef]
- Som, A.; Tezgel, A.O.; Gabriel, G.J.; Tew, G.N. Self-activation in de novo designed mimics of cell-penetrating peptides. Angew. Chem. 2011, 50, 6147–6150. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Reuter, A.; Tew, G.N. Protein transduction domain mimics: The role of aromatic functionality. Angew. Chem. 2012, 51, 980–983. [Google Scholar] [CrossRef] [PubMed]
- Terrasso, A.P.; Silva, A.C.; Filipe, A.; Pedroso, P.; Ferreira, A.L.; Alves, P.M.; Brito, C. Human neuron-astrocyte 3D co-culture-based assay for evaluation of neuroprotective compounds. J. Pharmacol. Toxicol. Methods 2017, 83, 72–79. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, T.; Liang, S.; Mu, W.; Fu, S.; Liu, Y.; Yang, R.; Zhang, Z.; Liu, Y.; Zhang, N. Lymph Node Delivery Strategy Enables the Activation of Cytotoxic T Lymphocytes and Natural Killer Cells to Augment Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 22213–22224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Afjei, R.; Massoud, T.F.; Paulmurugan, R. Comparison of cell-based assays to quantify treatment effects of anticancer drugs identifies a new application for Bodipy-L-cystine to measure apoptosis. Sci. Rep. 2018, 8, 16363. [Google Scholar] [CrossRef] [PubMed]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S.; Abe, T.; Gotoh, J.; Fukuuchi, Y. Substrate-dependence of reduction of MTT: A tetrazolium dye differs in cultured astroglia and neurons. Neurochem. Int. 2002, 40, 441–448. [Google Scholar] [CrossRef]
- Brouillet, E.; Hyman, B.T.; Jenkins, B.G.; Henshaw, D.R.; Schulz, J.B.; Sodhi, P.; Rosen, B.R.; Beal, M.F. Systemic or local administration of azide produces striatal lesions by an energy impairment-induced excitotoxic mechanism. Exp. Neurol. 1994, 129, 175–182. [Google Scholar] [CrossRef]
- Gao, C.; Chang, P.; Yang, L.; Wang, Y.; Zhu, S.; Shan, H.; Zhang, M.; Tao, L. Neuroprotective effects of hydrogen sulfide on sodium azide-induced oxidative stress in PC12 cells. Int. J. Mol. Med. 2018, 41, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Knyihar-Csillik, E.; Okuno, E.; Vecsei, L. Effects of in vivo sodium azide administration on the immunohistochemical localization of kynurenine aminotransferase in the rat brain. Neuroscience 1999, 94, 269–277. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Riluzole But Not Melatonin Ameliorates Acute Motor Neuron Degeneration and Moderately Inhibits SOD1-Mediated Excitotoxicity Induced Disrupted Mitochondrial Ca2+ Signaling in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2016, 10, 295. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Z.; Yuan, M.; Hassen, G.W.; Gampel, M.; Bergold, P.J. Lactate induced excitotoxicity in hippocampal slice cultures. Exp. Neurol. 2004, 186, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Guevara, P.; Sotelo, J. Influence of in vitro lactic acidosis on central nervous system neurons. Pathol. Biol. 1989, 37, 725–729. [Google Scholar]
- Goldman, S.A.; Pulsinelli, W.A.; Clarke, W.Y.; Kraig, R.P.; Plum, F. The effects of extracellular acidosis on neurons and glia in vitro. J. Cereb. Blood Flow Metab. 1989, 9, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Meade, A.J.; Meloni, B.P.; Mastaglia, F.L.; Watt, P.M.; Knuckey, N.W. AP-1 inhibitory peptides attenuate in vitro cortical neuronal cell death induced by kainic acid. Brain Res. 2010, 1360, 8–16. [Google Scholar] [CrossRef]
- Lariosa-Willingham, K.D.; Rosler, E.S.; Tung, J.S.; Dugas, J.C.; Collins, T.L.; Leonoudakis, D. A high throughput drug screening assay to identify compounds that promote oligodendrocyte differentiation using acutely dissociated and purified oligodendrocyte precursor cells. BMC Res. Notes 2016, 9, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Gautam, P.; Wennerberg, K.; Aittokallio, T. A normalized drug response metric improves accuracy and consistency of anticancer drug sensitivity quantification in cell-based screening. Commun. Biol. 2020, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Owens, B. Stroke. Nature 2014, 510, S1. [Google Scholar] [CrossRef] [Green Version]
- Venkat, P.; Shen, Y.; Chopp, M.; Chen, J. Cell-based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology 2018, 134, 310–322. [Google Scholar] [CrossRef]
- Chandra, A.; Stone, C.R.; Du, X.; Li, W.A.; Huber, M.; Bremer, R.; Geng, X.; Ding, Y. The cerebral circulation and cerebrovascular disease III: Stroke. Brain Circ. 2017, 3, 66–77. [Google Scholar]
- Xu, J.K.; Khan, A.R.; Fu, M.F.; Wang, R.J.; Ji, J.B.; Zhai, G.X. Cell-penetrating peptide: A means of breaking through the physiological barriers of different tissues and organs. J. Control. Release 2019, 309, 106–124. [Google Scholar] [CrossRef]
- Meng, T.; Cao, Q.; Lei, P.; Bush, A.I.; Xiang, Q.; Su, Z.; He, X.; Rogers, J.T.; Chiu, I.M.; Zhang, Q.; et al. Tat-haFGF14-154 Upregulates ADAM10 to Attenuate the Alzheimer Phenotype of APP/PS1 Mice through the PI3K-CREB-IRE1alpha/XBP1 Pathway. Mol. Ther. Nucleic Acids 2017, 7, 439–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, A.B.; Cross, J.L.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. Poly-arginine R18 and R18D (D-enantiomer) peptides reduce infarct volume and improves behavioural outcomes following perinatal hypoxic-ischaemic encephalopathy in the P7 rat. Mol. Brain 2018, 11, 8. [Google Scholar] [CrossRef]
- Chassagnon, I.R.; McCarthy, C.A.; Chin, Y.K.; Pineda, S.S.; Keramidas, A.; Mobli, M.; Pham, V.; De Silva, T.M.; Lynch, J.W.; Widdop, R.E.; et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2017, 114, 3750–3755. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.T. HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr. Drug Targets 2016, 17, 4–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaul, M.; Garden, G.A.; Lipton, S.A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001, 410, 988–994. [Google Scholar] [CrossRef]
- Haughey, N.J.; Nath, A.; Mattson, M.P.; Slevin, J.T.; Geiger, J.D. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J. Neurochem. 2001, 78, 457–467. [Google Scholar] [CrossRef]
- Valcour, V.; Shiramizu, B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion 2004, 4, 119–129. [Google Scholar] [CrossRef]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter Study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [Green Version]
- Chiu, L.S.; Anderton, R.S.; Cross, J.L.; Clark, V.W.; Edwards, A.B.; Knuckey, N.W.; Meloni, B.P. Assessment of R18, COG1410, and APP96-110 in Excitotoxicity and Traumatic Brain Injury. Transl. Neurosci. 2017, 8, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Di Menna, L.; Molinaro, G.; Di Nuzzo, L.; Riozzi, B.; Zappulla, C.; Pozzilli, C.; Turrini, R.; Caraci, F.; Copani, A.; Battaglia, G.; et al. Fingolimod protects cultured cortical neurons against excitotoxic death. Pharmacol. Res. 2013, 67, 1–9. [Google Scholar] [CrossRef]
- Cipriani, R.; Chara, J.C.; Rodriguez-Antiguedad, A.; Matute, C. FTY720 attenuates excitotoxicity and neuroinflammation. J. Neuroinflammation 2015, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- De Hemptinne, A.; Marrannes, R.; Vanheel, B. Influence of organic acids on intracellular pH. Am. J. Physiol. 1983, 245, C178–C183. [Google Scholar] [CrossRef]
- Baumann, F.; Leukel, P.; Doerfelt, A.; Beier, C.P.; Dettmer, K.; Oefner, P.J.; Kastenberger, M.; Kreutz, M.; Nickl-Jockschat, T.; Bogdahn, U.; et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 2009, 11, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, X.; Dai, D.; Song, X.; Xu, W. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters. Oncotarget 2016, 7, 23141–23155. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.Y.; Lee, T.J. Arginine synthesis from citrulline in perivascular nerves of cerebral artery. J. Pharmacol. Exp. Ther. 1995, 273, 895–901. [Google Scholar] [PubMed]
- Wang, T.Y.; Sun, Y.; Muthukrishnan, N.; Erazo-Oliveras, A.; Najjar, K.; Pellois, J.P. Membrane Oxidation Enables the Cytosolic Entry of Polyarginine Cell-penetrating Peptides. J. Biol. Chem. 2016, 291, 7902–7914. [Google Scholar] [CrossRef] [Green Version]
- Cerrato, C.P.; Pirisinu, M.; Vlachos, E.N.; Langel, U. Novel cell-penetrating peptide targeting mitochondria. FASEB J. 2015, 29, 4589–4599. [Google Scholar] [CrossRef]
- Ziegler, A.; Seelig, J. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: Binding mechanism and thermodynamic parameters. Biophys. J. 2004, 86, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Youn, J.I.; Park, S.H.; Jin, H.T.; Lee, C.G.; Seo, S.H.; Song, M.Y.; Lee, C.W.; Sung, Y.C. Enhanced delivery efficiency of recombinant adenovirus into tumor and mesenchymal stem cells by a novel PTD. Cancer Gene Ther. 2008, 15, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, Q.; Liu, X.; Hu, W.; Wang, Y. Neuroprotective effect of TAT-14-3-3epsilon fusion protein against cerebral ischemia/reperfusion injury in rats. PLoS ONE 2014, 9, e93334. [Google Scholar]
- Tu, J.; Zhang, X.; Zhu, Y.; Dai, Y.; Li, N.; Yang, F.; Zhang, Q.; Brann, D.W.; Wang, R. Cell-Permeable Peptide Targeting the Nrf2-Keap1 Interaction: A Potential Novel Therapy for Global Cerebral Ischemia. J. Neurosci. 2015, 35, 14727–14739. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Pei, W.; Ge, H.; Liang, Q.; Luo, Y.; Sharp, F.R.; Lu, A.; Ran, R.; Graham, S.H.; Chen, J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J. Neurosci. 2002, 22, 5423–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilic, E.; Kilic, U.; Hermann, D.M. TAT-GDNF in neurodegeneration and ischemic stroke. CNS Drug Rev. 2005, 11, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Doeppner, T.R.; Kaltwasser, B.; Fengyan, J.; Hermann, D.M.; Bahr, M. TAT-Hsp70 induces neuroprotection against stroke via anti-inflammatory actions providing appropriate cellular microenvironment for transplantation of neural precursor cells. J. Cereb. Blood Flow Metab. 2013, 33, 1778–1788. [Google Scholar] [CrossRef] [Green Version]
- Kilic, U.; Kilic, E.; Dietz, G.P.; Bahr, M. The TAT protein transduction domain enhances the neuroprotective effect of glial-cell-line-derived neurotrophic factor after optic nerve transection. Neuro Degener. Dis. 2004, 1, 44–49. [Google Scholar] [CrossRef]
- Pei, D.S.; Wang, X.T.; Liu, Y.; Sun, Y.F.; Guan, Q.H.; Wang, W.; Yan, J.Z.; Zong, Y.Y.; Xu, T.L.; Zhang, G.Y. Neuroprotection against ischaemic brain injury by a GluR6-9c peptide containing the TAT protein transduction sequence. Brain 2006, 129, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popiel, H.A.; Nagai, Y.; Fujikake, N.; Toda, T. Protein transduction domain-mediated delivery of QBP1 suppresses polyglutamine-induced neurodegeneration in vivo. Mol. Ther. J. Am. Soc. Gene Ther. 2007, 15, 303–309. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Graslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herve, F.; Ghinea, N.; Scherrmann, J.M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Meloni, B.P.; Mastaglia, F.L.; Knuckey, N.W. Cationic Arginine-Rich Peptides (CARPs): A Novel Class of Neuroprotective Agents with a Multimodal Mechanism of Action. Front Neurol 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Kwok, S.J.; Lombardi, J.; Turro, N.J.; Eisenthal, K.B. Label-free probe of HIV-1 TAT peptide binding to mimetic membranes. Proc. Natl. Acad. Sci. USA 2014, 111, 12684–12688. [Google Scholar] [CrossRef] [Green Version]
- Chiu, L.S.; Anderton, R.S.; Knuckey, N.W.; Meloni, B.P. The neuroprotective potential of arginine-rich peptides for the acute treatment of traumatic brain injury. Expert Rev. Neurother. 2016, 16, 361–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorents, A.; Kodavali, P.K.; Oskolkov, N.; Langel, U.; Hallbrink, M.; Pooga, M. Cell-penetrating Peptides Split into Two Groups Based on Modulation of Intracellular Calcium Concentration. J. Biol. Chem. 2012, 287, 16880–16889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, A.; Psooy, K.; Martin, C.; Knudsen, B.; Magnuson, D.S.K.; Haughey, N.; Geiger, J.D. Identification of a human immunodeficiency virus type 1 tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 1996, 70, 1475–1480. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Molina, B.; Lick, A.N.; Nasrolahi Shirazi, A.; Oh, D.; Tiwari, R.; El-Sayed, N.S.; Parang, K.; Lindberg, I. Cationic Cell-Penetrating Peptides Are Potent Furin Inhibitors. PLoS ONE 2015, 10, e0130417. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [Green Version]
- Debiec, K.T.; Cerutti, D.S.; Baker, L.R.; Gronenborn, A.M.; Case, D.A.; Chong, L.T. Further along the Road Less Traveled: AMBER ff15ipq, an Original Protein Force Field Built on a Self-Consistent Physical Model. J. Chem. Theory Comput. 2016, 12, 3926–3947. [Google Scholar] [CrossRef]
- Tsui, V.; Case, D.A. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers 2000, 56, 275–291. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., 3rd. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Koradi, R.; Billeter, M.; Wuthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef]








| Peptide | Sequence | Remarks | Molecular Weight [g/mol] | Net Chargeat pH 7.0 |
|---|---|---|---|---|
| Tat(49–57)-NH2 | RKKR52RQRRR57-amide | Native sequence | 1338.62 | 9 |
| DR52 | RKKrRQRRR-amide | 1338.62 | 9 | |
| Tat6s | Ac-RRQRRR-amide | Ac-Tat(52–57)-NH2 | 968.13 | 5 |
| Tat7s | Ac-R(Me)2RQRRR-amide (ASDM) * | Ac-[Arg(Me)2]Tat(52–57)-NH2 | 996.13 | 5 |
| PTD4 | YARAAARQARA-amide | 1203.36 | 4 | |
| Prop-Tat | propiolyl-RKKRRQRRR57-amide | Prop-Tat(49–57)-NH2 | 1390.62 | 8 |
| TP10 | AGYLLGKINLKALAALAKKIL-amide | 2181.75 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazuryk, J.; Puchalska, I.; Koziński, K.; Ślusarz, M.J.; Ruczyński, J.; Rekowski, P.; Rogujski, P.; Płatek, R.; Wiśniewska, M.B.; Piotrowski, A.; et al. PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 6086. https://doi.org/10.3390/ijms22116086
Mazuryk J, Puchalska I, Koziński K, Ślusarz MJ, Ruczyński J, Rekowski P, Rogujski P, Płatek R, Wiśniewska MB, Piotrowski A, et al. PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke. International Journal of Molecular Sciences. 2021; 22(11):6086. https://doi.org/10.3390/ijms22116086
Chicago/Turabian StyleMazuryk, Jarosław, Izabela Puchalska, Kamil Koziński, Magdalena J. Ślusarz, Jarosław Ruczyński, Piotr Rekowski, Piotr Rogujski, Rafał Płatek, Marta Barbara Wiśniewska, Arkadiusz Piotrowski, and et al. 2021. "PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke" International Journal of Molecular Sciences 22, no. 11: 6086. https://doi.org/10.3390/ijms22116086
APA StyleMazuryk, J., Puchalska, I., Koziński, K., Ślusarz, M. J., Ruczyński, J., Rekowski, P., Rogujski, P., Płatek, R., Wiśniewska, M. B., Piotrowski, A., Janus, Ł., Skowron, P. M., Pikuła, M., Sachadyn, P., Rodziewicz-Motowidło, S., Czupryn, A., & Mucha, P. (2021). PTD4 Peptide Increases Neural Viability in an In Vitro Model of Acute Ischemic Stroke. International Journal of Molecular Sciences, 22(11), 6086. https://doi.org/10.3390/ijms22116086