The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study
Abstract
:1. Introduction
2. Results
2.1. PAG Improves Age-Related Leaky Gut Condition
2.2. PAG Increases Tight Junction Proteins of Intestinal Epithelial Cells
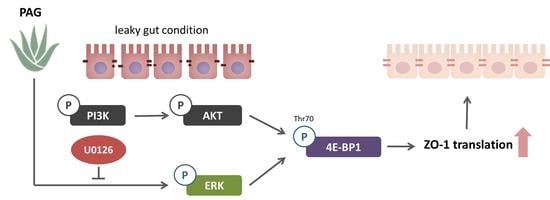
2.3. PAG Conserves Intestinal Tight Junction at Translation Level by Deactivating 4E-BP1 via 115 MAPK/ERK Signaling Pathway 116
3. Discussion
4. Materials and Methods
4.1. Animals, Cells, and Materials
4.2. Assessment of the Intestinal Barrier—L/M Test and Teer Measurement
4.3. Western Blot Analysis
4.4. Polymerase Chain Reaction (PCR)
4.5. Immunofluorescence Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Hollander, D. Intestinal permeability, leaky gut, and intestinal disorders. Curr. Gastroenterol. Rep. 1999, 1, 410–416. [Google Scholar] [CrossRef]
- Galland, L. Leaky gut syndromes: Breaking the vicious cycle. Townsend Lett. Dr. 1995, 145, 63–68. [Google Scholar]
- Lipski, E. Leaky Gut Syndrome; McGraw Hill Professional: New York, NY, USA, 1998. [Google Scholar]
- Dehner, C.; Fine, R.; Kriegel, M.A. The Microbiome in Systemic Autoimmune Disease–Mechanistic Insights from Recent Studies. Curr. Opin. Rheumatol. 2019, 31, 201. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Kirby, J.; Reilly, C.M.; Luo, X.M. Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 2017, 8, 598. [Google Scholar] [CrossRef] [Green Version]
- Quigley, E.M. Leaky gut–concept or clinical entity? Curr. Opin. Gastroenterol. 2016, 32, 74–79. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera—A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 2019, 59, S244–S256. [Google Scholar] [CrossRef]
- Ni, Y.; Turner, D.; Yates, K.Á.; Tizard, I. Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int. Immunopharmacol. 2004, 4, 1745–1755. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Li, F.; Du, P.; Yang, W.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the seeds of Plantago asiatica L. alleviates nonylphenol induced intestinal barrier injury by regulating tight junctions in human Caco-2 cell line. Int. J. Biol. Macromol. 2020, 164, 2134–2140. [Google Scholar] [CrossRef]
- Zuo, T.; Cao, L.; Li, X.; Zhang, Q.; Xue, C.; Tang, Q. The squid ink polysaccharides protect tight junctions and adherens junctions from chemotherapeutic injury in the small intestinal epithelium of mice. Nutr. Cancer 2015, 67, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Joseph, T.P.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A polysaccharide isolated from Dictyophora indusiata promotes recovery from antibiotic-driven intestinal dysbiosis and improves gut epithelial barrier function in a mouse model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [Green Version]
- Ying, M.; Zheng, B.; Yu, Q.; Hou, K.; Wang, H.; Zhao, M.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Ganoderma atrum polysaccharide ameliorates intestinal mucosal dysfunction associated with autophagy in immunosuppressed mice. Food Chem. Toxicol. 2020, 138, 111244. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, J.-H.; Bai, G.; Shen, G.-S.; Chen, J.; Liu, J.-N.; Wang, S.; Liu, X.-J. Acanthopanax senticosus polysaccharides-induced intestinal tight junction injury alleviation via inhibition of NF-κB/MLCK pathway in a mouse endotoxemia model. World J. Gastroenterol. 2017, 23, 2175. [Google Scholar] [CrossRef]
- Yue, Y.; Wu, S.; Li, Z.; Li, J.; Li, X.; Xiang, J.; Ding, H. Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct. 2015, 6, 2568–2577. [Google Scholar] [CrossRef]
- Vojdani, A. For the assessment of intestinal permeability, size matters. Altern. Health Med 2013, 19, 12–24. [Google Scholar]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gingras, A.-C.; Kennedy, S.G.; O’Leary, M.A.; Sonenberg, N.; Hay, N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt (PKB) signaling pathway. Genes Dev. 1998, 12, 502–513. [Google Scholar] [CrossRef]
- Azar, R.; Najib, S.; Lahlou, H.; Susini, C.; Pyronnet, S. Phosphatidylinositol 3-kinase-dependent transcriptional silencing of the translational repressor 4E-BP1. Cell. Mol. Life Sci. 2008, 65, 3110–3117. [Google Scholar] [CrossRef]
- Rojo, F.; Najera, L.; Lirola, J.; Jiménez, J.; Guzmán, M.; Sabadell, M.D.; Baselga, J.; y Cajal, S.R. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin. Cancer Res. 2007, 13, 81–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, S.-A.; Oh, S.-T.; Song, S.; Kim, M.-R.; Kim, D.-S.; Woo, S.-S.; Jo, T.H.; Park, Y.I.; Lee, C.-K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef]
- Kim, M.W.; Kang, J.-H.; Shin, E.; Shim, K.-S.; Kim, M.J.; Lee, C.-K.; Yoon, Y.S.; Oh, S.H. Processed Aloe vera gel attenuates non-steroidal anti-inflammatory drug (NSAID)-induced small intestinal injury by enhancing mucin expression. Food Funct. 2019, 10, 6088–6097. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Lkhagva-Yondon, E.; Kim, M.; Lim, Y.R.; Shin, E.; Lee, C.K.; Jeon, M.S. Oral treatment with Aloe polysaccharide ameliorates ovalbumin-induced atopic dermatitis by restoring tight junctions in skin. Scand. J. Immunol. 2019, 91, e12856. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Budai, M.M.; Varga, A.; Milesz, S.; Tőzsér, J.; Benkő, S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol. Immunol. 2013, 56, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, purification, structural characteristics, biological activities and pharmacological applications of acemannan, a polysaccharide from aloe vera: A review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 729–756. [Google Scholar] [CrossRef] [Green Version]
- Sumi, K.; Higashi, S.; Natsume, M.; Kawahata, K.; Nakazato, K. Temporal changes in ERK phosphorylation are harmonious with 4E-BP1, but not p70S6K, during clenbuterol-induced hypertrophy in the rat gastrocnemius. Appl. Physiol. Nutr. Metab. 2014, 39, 902–910. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Q.; She, Q.-B. New insights into 4E-BP1-regulated translation in cancer progression and metastasis. Cancer Cell Microenviron. 2014, 1, e311. [Google Scholar]
- Bhandari, B.K.; Feliers, D.; Duraisamy, S.; Stewart, J.L.; Gingras, A.-C.; Abboud, H.E.; Choudhury, G.G.; Sonenberg, N.; Kasinath, B.S. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001, 59, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, T.P.; Tee, A.R.; Proud, C.G. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J. Biol. Chem. 2002, 277, 11591–11596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, Y.; Azar, R.; Bousquet, C.; Pyronnet, S. Anti-oncogenic potential of the eIF4E-binding proteins. Oncogene 2013, 32, 671–677. [Google Scholar] [CrossRef]
- Gingras, A.-C.; Raught, B.; Gygi, S.P.; Niedzwiecka, A.; Miron, M.; Burley, S.K.; Polakiewicz, R.D.; Wyslouch-Cieszynska, A.; Aebersold, R.; Sonenberg, N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001, 15, 2852–2864. [Google Scholar]
- Wang, X.; Li, W.; Parra, J.-L.; Beugnet, A.; Proud, C.G. The C terminus of initiation factor 4E-binding protein 1 contains multiple regulatory features that influence its function and phosphorylation. Mol. Cell. Biol. 2003, 23, 1546–1557. [Google Scholar] [CrossRef] [Green Version]
- Haasbroek, A.; Willers, C.; Glyn, M.; Du Plessis, L.; Hamman, J. Intestinal drug absorption enhancement by Aloe vera gel and whole leaf extract: In vitro investigations into the mechanisms of action. Pharmaceutics 2019, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Lu, Z.; Viljoen, A.; Hamman, J. Intestinal drug transport enhancement by Aloe vera. Planta Med. 2009, 75, 587–595. [Google Scholar] [CrossRef]
- Beneke, C.; Viljoen, A.; Hamman, J. In vitro drug absorption enhancement effects of Aloe vera and Aloe ferox. Sci. Pharm. 2012, 80, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Lebitsa, T.; Viljoen, A.; Lu, Z.; Hamman, J. In vitro drug permeation enhancement potential of aloe gel materials. Curr. Drug Deliv. 2012, 9, 297–304. [Google Scholar] [CrossRef]
- Vinson, J.A.; Al Kharrat, H.; Andreoli, L. Effect of Aloe vera preparations on the human bioavailability of vitamins C and E. Phytomedicine 2005, 12, 760–765. [Google Scholar] [CrossRef]
- Irvine, E.J.; Marshall, J.K. Increased intestinal permeability precedes the onset of Crohn’s disease in a subject with familial risk. Gastroenterology 2000, 119, 1740–1744. [Google Scholar] [CrossRef]
- Thjodleifsson, B.; Sigthorsson, G.; Cariglia, N.; Reynisdottir, I.; Gudbjartsson, D.F.; Kristjansson, K.; Meddings, J.B.; Gudnason, V.; Wandall, J.H.; Andersen, L.P. Subclinical intestinal inflammation: An inherited abnormality in Crohn’s disease relatives? Gastroenterology 2003, 124, 1728–1737. [Google Scholar] [CrossRef]
- Montalto, M.; Curigliano, V.; Santoro, L.; Armuzzi, A.; Cammarota, G.; Covino, M.; Mentella, M.C.; Ancarani, F.; Manna, R.; Gasbarrini, A. Fecal calprotectin in first-degree relatives of patients with ulcerative colitis. Am. J. Gastroenterol. 2007, 102, 132–136. [Google Scholar] [CrossRef]
- Madsen, K.L.; Malfair, D.; Gray, D.; Doyle, J.S.; Jewell, L.D.; Fedorak, R.N. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm. Bowel Dis. 1999, 5, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Langmead, L.; Feakins, R.; Goldthorpe, S.; Holt, H.; Tsironi, E.; De Silva, A.; Jewell, D.; Rampton, D. Randomized, double-blind, placebo-controlled trial of oral Aloe vera gel for active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 19, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Volynets, V.; Reichold, A.; Bárdos, G.; Rings, A.; Bleich, A.; Bischoff, S.C. Assessment of the intestinal barrier with five different permeability tests in healthy C57BL/6J and BALB/cJ mice. Dig. Dis. Sci. 2016, 61, 737–746. [Google Scholar] [CrossRef]
- Maeng, H.-J.; Yoon, J.-H.; Chun, K.-H.; Kim, S.T.; Jang, D.-J.; Park, J.-E.; Kim, Y.H.; Kim, S.-B.; Kim, Y.C. Metabolic stability of D-allulose in biorelevant media and hepatocytes: Comparison with fructose and erythritol. Foods 2019, 8, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.W.; Choi, S.; Kim, S.Y.; Yoon, Y.S.; Kang, J.-H.; Oh, S.H. Allyl Isothiocyanate Ameliorates Dextran Sodium Sulfate-Induced Colitis in Mouse by Enhancing Tight Junction and Mucin Expression. Int. J. Mol. Sci. 2018, 19, 2025. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Phan, T.H.; Park, S.Y.; Jung, H.J.; Kim, M.W.; Cho, E.; Shim, K.-S.; Shin, E.; Yoon, J.-H.; Maeng, H.-J.; Kang, J.-H.; et al. The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study. Int. J. Mol. Sci. 2021, 22, 6515. https://doi.org/10.3390/ijms22126515
Le Phan TH, Park SY, Jung HJ, Kim MW, Cho E, Shim K-S, Shin E, Yoon J-H, Maeng H-J, Kang J-H, et al. The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study. International Journal of Molecular Sciences. 2021; 22(12):6515. https://doi.org/10.3390/ijms22126515
Chicago/Turabian StyleLe Phan, Thu Han, Se Yong Park, Hyun Jin Jung, Min Woo Kim, Eunae Cho, Kyu-Suk Shim, Eunju Shin, Jin-Ha Yoon, Han-Joo Maeng, Ju-Hee Kang, and et al. 2021. "The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study" International Journal of Molecular Sciences 22, no. 12: 6515. https://doi.org/10.3390/ijms22126515
APA StyleLe Phan, T. H., Park, S. Y., Jung, H. J., Kim, M. W., Cho, E., Shim, K. -S., Shin, E., Yoon, J. -H., Maeng, H. -J., Kang, J. -H., & Oh, S. H. (2021). The Role of Processed Aloe vera Gel in Intestinal Tight Junction: An In Vivo and In Vitro Study. International Journal of Molecular Sciences, 22(12), 6515. https://doi.org/10.3390/ijms22126515