Proteus mirabilis Urease: Unsuspected Non-Enzymatic Properties Relevant to Pathogenicity
Abstract
:1. Introduction
2. Results
2.1. Moonlighting (Non-Enzymatic) Properties of PMU
2.1.1. Aggregation of Human Platelets by PMU and Its Subunits
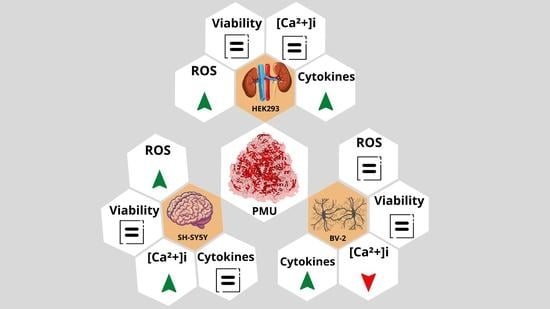
2.1.2. Effects of PMU in Cell Cultures
2.1.3. Pro-Inflammatory Properties of PMU in HEK293 Cells
2.1.4. Pro-Inflammatory Potential of PMU in CNS-Derived Cells
2.1.5. Modulation by PMU of Intracellular Calcium Levels
2.2. Internalization and Nuclear Localization of PMU
3. Materials and Methods
3.1. Plasmid Construction and Bacterial Strain
3.2. Bacterial Growth and Induction of Proteus Mirabilis Urease
3.3. Crude Extract and Purification of PMU
3.4. Protein Determination
3.5. SDS-PAGE
3.6. Urease Assay
3.7. Platelet Aggregation
Platelet Aggregation by Turbidimetry
3.8. Cell Cultures
3.9. Cellular Viability
3.10. Intracellular Levels of Reactive Oxygen Species
3.11. Determination of Intracellular Ca2+ Levels
3.12. Cytokines Determination
3.13. Fluorescence Microscopy, Texas Red-Labeled PMU and Interaction with Cells
3.14. Analysis of PMU for Nuclear Localization Sequences
3.15. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armbruster, C.E.; Mobley, H.L.T.; Pearson, M.M. Pathogenesis of Proteus mirabilis Infection. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Nicolle, L.E. Urinary catheter-associated infections. Infect. Dis. Clin. N. Am. 2012, 26, 13–27. [Google Scholar] [CrossRef]
- Jones, B.D.; Lockatell, C.V.; Johnson, D.E.; Warren, J.W.; Mobley, H.L. Construction of a urease-negative mutant of Proteus mirabilis: Analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 1990, 58, 1120–1123. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, J.N.; Norsworthy, A.N.; Sun, T.T.; Pearson, M.M. Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc. Natl. Acad. Sci. USA 2016, 113, 4494–4499. [Google Scholar] [CrossRef] [Green Version]
- Griffith, D.P.; Musher, D.M.; Itin, C. Urease. The primary cause of infection-induced urinary stones. Investig. Urol. 1976, 13, 346–350. [Google Scholar]
- Coker, C.; Poore, C.A.; Li, X.; Mobley, H.L. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2000, 2, 1497–1505. [Google Scholar] [CrossRef]
- Schiwon, M.; Weisheit, C.; Franken, L.; Gutweiler, S.; Dixit, A.; Meyer-Schwesinger, C.; Pohl, J.M.; Maurice, N.J.; Thiebes, S.; Lorenz, K.; et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 2014, 156, 456–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, H.; Mori, M.; Suzuki, M.; Sakurai, K.; Miura, S.; Ishii, H. Extensive DNA damage induced by monochloramine in gastric cells. Cancer Lett. 1997, 115, 243–248. [Google Scholar] [CrossRef]
- Dzutsev, A.; Trinchieri, G. Proteus mirabilis: The Enemy Within. Immunity 2015, 42, 602–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.; Lehman, D. Cerebral abscess complicating Proteus mirabilis meningitis in a newborn infant. J. Child Neurol. 2012, 27, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Senior, B.W.; Sweeney, G. The association of particular types of Proteus with chronic suppurative otitis media. J. Med. Microbiol. 1984, 17, 201–205. [Google Scholar] [CrossRef]
- Chang, W.N.; Tsai, Y.C.; Chien, C.C.; Huang, C.R.; Lu, C.H. Frequent association with neurosurgical conditions in adult Proteus mirabilis meningitis: Report of five cases. Clin. Neurol. Neurosurg. 2002, 104, 121–124. [Google Scholar] [CrossRef]
- Ebringer, A.; Rashid, T. Rheumatoid arthritis is caused by a Proteus urinary tract infection. APMIS 2014, 122, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Samtoy, B.; Debeukelaer, M.M. Ammonia encephalopathy secondary to urinary tract infection with Proteus mirabilis. Pediatrics 1980, 65, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, N.; Ju, I.G.; Eo, H.; Lim, S.M.; Jang, S.E.; Kim, D.H.; Oh, M.S. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 2018, 8, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Wu, X.L.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.F.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Ebringer, A.; Ahmadi, K.; Wrigglesworth, J.; Tiwana, H.; Fielder, M.; Binder, A.; Ettelaie, C.; Cunningham, P.; Joannou, C.; et al. Shared amino acid sequences between major histocompatibility complex class II glycoproteins, type XI collagen and Proteus mirabilis in rheumatoid arthritis. Ann. Rheum. Dis. 1995, 54, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsan, N.; Ahmad, S.; Azam, S.S.; Rungrotmongkol, T.; Uddin, R. Proteome-wide identification of epitope-based vaccine candidates against multi-drug resistant Proteus mirabilis. Biologicals 2018, 55, 27–37. [Google Scholar] [CrossRef]
- Norsworthy, A.N.; Pearson, M.M. From Catheter to Kidney Stone: The Uropathogenic Lifestyle of Proteus mirabilis. Trends Microbiol. 2017, 25, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rego, Y.F.; Queiroz, M.P.; Brito, T.O.; Carvalho, P.G.; de Queiroz, V.T.; de Fatima, A.; Macedo, F., Jr. A review on the development of urease inhibitors as antimicrobial agents against pathogenic bacteria. J. Adv. Res. 2018, 13, 69–100. [Google Scholar] [CrossRef]
- Ligabue-Braun, R.; Andreis, F.C.; Verli, H.; Carlini, C.R. 3-to-1: Unraveling structural transitions in ureases. Naturwissenschaften 2013, 100, 459–467. [Google Scholar] [CrossRef]
- Island, M.D.; Mobley, H.L. Proteus mirabilis urease: Operon fusion and linker insertion analysis of ure gene organization, regulation, and function. J. Bacteriol. 1995, 177, 5653–5660. [Google Scholar] [CrossRef] [Green Version]
- Ligabue-Braun, R.; Carlini, C.R. Moonlighting toxins: Ureases and beyond. Toxinology 2017, 199–219. [Google Scholar] [CrossRef]
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects, catalytic, and non-catalytic properties—A review. J. Adv. Res. 2018, 13, 3–17. [Google Scholar] [CrossRef]
- Follmer, C.; Barcellos, G.B.; Zingali, R.B.; Machado, O.L.T.; Alves, E.W.; Barja-Fidalgo, C.; Guimaraes, J.A.; Carlini, C.R. Canatoxin, a toxic protein from jack beans (Canavalia ensiformis), is a variant form of urease (EC 3.5.1.5): Biological effects of urease independent of its ureolytic activity. Biochem. J. 2001, 360, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Barja-Fidalgo, C.; Guimaraes, J.A.; Carlini, C.R. Lipoxygenase-mediated secretory effect of canatoxin the toxic protein from Canavalia ensiformis seeds. Toxicon 1991, 29, 453–459. [Google Scholar] [CrossRef]
- Barja-Fidalgo, C.; Carlini, C.R.; Guimaraes, J.A.; Flores, C.A.; Cunha, F.Q.; Ferreira, S.H. Role of resident macrophages in canatoxin-induced in vivo neutrophil migration. Inflammation 1992, 16, 1–12. [Google Scholar] [CrossRef]
- Benjamin, C.F.; Carlini, C.R.; Barja-Fidalgo, C. Pharmacological characterization of rat paw edema induced by canatoxin, the toxic protein from Canavalia ensiformis (jack bean) seeds. Toxicon 1992, 30, 879–885. [Google Scholar] [CrossRef]
- Carlini, C.R.; Oliveira, A.E.; Azambuja, P.; Xavier-Filho, J.; Wells, M.A. Biological effects of canatoxin in different insect models: Evidence for a proteolytic activation of the toxin by insect cathepsinlike enzymes. J. Econ. Entomol. 1997, 90, 340–348. [Google Scholar] [CrossRef]
- Oliveira, A.E.A.; Gomes, V.M.; Sales, M.P.; Fernandes, K.V.S.; Carlini, C.R.; Xavier-Filho, J. The toxicity of jack bean [Canavalia ensiformis (L.) DC.] canatoxin to plant pathogenic fungi. Rev. Bras. Biol. 1999, 59, 59–62. [Google Scholar] [CrossRef]
- Wassermann, G.E.; Olivera-Severo, D.; Uberti, A.F.; Carlini, C.R. Helicobacter pylori urease activates blood platelets through a lipoxygenase-mediated pathway. J. Cell. Mol. Med. 2010, 14, 2025–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scopel-Guerra, A.; Olivera-Severo, D.; Staniscuaski, F.; Uberti, A.F.; Callai-Silva, N.; Jaeger, N.; Porto, B.N.; Carlini, C.R. The impact of Helicobacter pylori urease upon platelets and consequent contributions to inflammation. Front. Microbiol. 2017, 8, 2447. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.J.; Moraes, J.A.; Silva, V.N.; Helal-Neto, E.; Uberti, A.F.; Scopel-Guerra, A.; Olivera-Severo, D.; Carlini, C.R.; Barja-Fidalgo, C. Helicobacter pylori urease induces pro-inflammatory effects and differentiation of human endothelial cells: Cellular and Molecular Mechanism. Helicobacter 2019, 24, e12573. [Google Scholar] [CrossRef] [PubMed]
- Uberti, A.F.; Olivera-Severo, D.; Wassermann, G.E.; Scopel-Guerra, A.; Moraes, J.A.; Barcellos-de-Souza, P.; Barja-Fidalgo, C.; Carlini, C.R. Pro-inflammatory properties and neutrophil activation by Helicobacter pylori urease. Toxicon 2013, 69, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Severo, D.; Uberti, A.F.; Marques, M.S.; Pinto, M.T.; Gomez-Lazaro, M.; Figueiredo, C.; Leite, M.; Carlini, C.R. A new role for Helicobacter pylori urease: Contributions to angiogenesis. Front. Microbiol. 2017, 8, e1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivera-Severo, D.; Wassermann, G.E.; Carlini, C.R. Bacillus pasteurii urease shares with plant ureases the ability to induce aggregation of blood platelets. Arch. Biochem. Biophys. 2006, 452, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Carlini, C.R.; Ligabue-Braun, R. Ureases as multifunctional toxic proteins: A review. Toxicon 2016, 110, 90–109. [Google Scholar] [CrossRef]
- Follmer, C.; Real-Guerra, R.; Wasserman, G.E.; Olivera-Severo, D.; Carlini, C.R. Jackbean, soybean and Bacillus pasteurii ureases - Biological effects unrelated to ureolytic activity. Eur. J. Biochem. 2004, 271, 1357–1363. [Google Scholar] [CrossRef]
- Carlini, C.R.; Guimaraes, J.A.; Ribeiro, J.M. Platelet release reaction and aggregation induced by canatoxin, a convulsant protein: Evidence for the involvement of the platelet lipoxygenase pathway. Br. J. Pharmacol. 1985, 84, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Suarez, I.; Bodega, G.; Fernandez, B. Glutamine synthetase in brain: Effect of ammonia. Neurochem. Int. 2002, 41, 123–142. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, S.H.; Baik, S.C.; Kim, D.R.; Park, J.Y.; Lee, Y.S.; Choi, C.H.; Lee, J.C. Prediction and screening of nuclear targeting proteins with nuclear localization signals in Helicobacter pylori. J. Microbiol. Meth. 2012, 91, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Hari, P.S.; Sridhar, T.S.; Kumar, R.P. NLScore: A novel quantitative algorithm based on 3 dimensional structural determinants to predict the probability of nuclear localization in proteins containing classical nuclear localization signals. J. Mol. Model. 2017, 23, 258. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.T.; Foxall, P.A.; Russell, R.; Mobley, H.L. Purification of recombinant Helicobacter pylori urease apoenzyme encoded by ureA and ureB. Infect. Immun. 1992, 60, 2657–2666. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, Y.; Li, S. Astaxanthin attenuates glutamate-induced apoptosis via inhibition of calcium influx and endoplasmic reticulum stress. Eur. J. Pharmacol. 2017, 806, 43–51. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, T.; Wachowicz, B.; Saluk-Juszczak, J.; Kaca, W. Polysaccharide part of Proteus mirabilis lipopolysaccharide may be responsible for the stimulation of platelet adhesion to collagen. Platelets 2002, 13, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.A.; Schultz, E.F.; Hay, S.N.; Brecher, M.E. Thrombotic thrombocytopenic purpura and urinary tract infections: Is there a connection? Am. J. Clin. Pathol. 2011, 135, 85–88. [Google Scholar] [CrossRef]
- Kourbeti, I.S.; Vakis, A.F.; Ziakas, P.; Karabetsos, D.; Potolidis, E.; Christou, S.; Samonis, G. Infections in patients undergoing craniotomy: Risk factors associated with post-craniotomy meningitis. J. Neurosurg. 2015, 122, 1113–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boertien, J.M.; Pereira, P.A.B.; Aho, V.T.E.; Scheperjans, F. Increasing Comparability and Utility of Gut Microbiome Studies in Parkinson’s Disease: A Systematic Review. J. Parkinsons’s Dis. 2019, 9, S297–S312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.M.; Rodrigo-Angulo, M.; et al. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.R.; Ernst, P.B.; Kawabata, S.; Kiyono, H.; Graham, M.F.; Smith, P.D. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J. Infect. Dis. 1998, 178, 1516–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.R.; Mobley, H.L.; Perez-Perez, G.I.; Blaser, M.J.; Smith, P.D. Helicobacter pylori urease is a potent stimulus of mononuclear phagocyte activation and inflammatory cytokine production. Gastroenterology 1996, 111, 419–425. [Google Scholar] [CrossRef]
- Tanahashi, T.; Kita, M.; Kodama, T.; Yamaoka, Y.; Sawai, N.; Ohno, T.; Mitsufuji, S.; Wei, Y.P.; Kashima, K.; Imanishi, J. Cytokine expression and production by purified Helicobacter pylori urease in human gastric epithelial cells. Infect. Immun. 2000, 68, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, K.N.; Hartung, M.L.; Urban, S.; Kyburz, A.; Bahlmann, A.S.; Lind, J.; Backert, S.; Taube, C.; Muller, A. Helicobacter urease–induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J. Clin. Investig. 2015, 125, 3297. [Google Scholar] [CrossRef]
- Renauld, A.E.; Spengler, R.N. Tumor necrosis factor expressed by primary hippocampal neurons and SH-SY5Y cells is regulated by alpha(2)-adrenergic receptor activation. J. Neurosci. Res. 2002, 67, 264–274. [Google Scholar] [CrossRef]
- Saeed, Y.; Xie, B.; Xu, J.; Wang, H.; Hassan, M.; Wang, R.; Hong, M.; Hong, Q.; Deng, Y. Indirect effects of radiation induce apoptosis and neuroinflammation in neuronal SH-SY5Y cells. Neurochem. Res. 2014, 39, 2334–2342. [Google Scholar] [CrossRef]
- Gomez-Santos, C.; Francisco, R.; Gimenez-Xavier, P.; Ambrosio, S. Dopamine induces TNFalpha and TNF-R1 expression in SH-SY5Y human neuroblastoma cells. Neuroreport 2007, 18, 1725–1728. [Google Scholar] [CrossRef]
- Ghazaleh, F.A.; Francischetti, I.M.; Gombarovits, M.E.; Carlini, C.R. Stimulation of calcium influx and platelet activation by canatoxin: Methoxyverapamil inhibition and downregulation by cGMP. Arch. Biochem. Biophys 1997, 339, 362–367. [Google Scholar] [CrossRef] [PubMed]
- An Haack, K.; Narayan, S.B.; Li, H.; Warnock, A.; Tan, L.; Bennett, M.J. Screening for calcium channel modulators in CLN3 siRNA knock down SH-SY5Y neuroblastoma cells reveals a significant decrease of intracellular calcium levels by selected L-type calcium channel blockers. Biochim. Biophys. Acta 2011, 1810, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, L.S.; Dos Santos, N.A.G.; Emerick, G.L.; Santos, A.C.D. L- and T-type calcium channel blockers protect against the inhibitory effects of mipafox on neurite outgrowth and plasticity-related proteins in SH-SY5Y cells. J. Toxicol. Environ. Health A 2017, 80, 1086–1097. [Google Scholar] [CrossRef]
- Berjukow, S.; Doring, F.; Froschmayr, M.; Grabner, M.; Glossmann, H.; Hering, S. Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br. J. Pharmacol. 1996, 118, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Skopin, A.; Shalygin, A.; Vigont, V.; Zimina, O.; Glushankova, L.; Mozhayeva, G.N.; Kaznacheyeva, E. TRPC1 protein forms only one type of native store-operated channels in HEK293 cells. Biochimie 2013, 95, 347–353. [Google Scholar] [CrossRef]
- Shalygin, A.; Skopin, A.; Kalinina, V.; Zimina, O.; Glushankova, L.; Mozhayeva, G.N.; Kaznacheyeva, E. STIM1 and STIM2 proteins differently regulate endogenous store-operated channels in HEK293 cells. J. Biol. Chem. 2015, 290, 4717–4727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, K.; Kettenmann, H. Functional role of calcium signals for microglial function. Glia 2006, 54, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, M.M.; Carlini, C.R.; Guimaraes, J.A.; Marquessilva, V.M.; Rumjanek, V.M. Effect of Canatoxin on Cell-Cultures. Cell Biol. Int. Rep. 1991, 15, 581–594. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, S.H.; Kim, J.M.; Baik, S.C.; Lee, J.C. Morphological changes in human gastric epithelial cells induced by nuclear targeting of Helicobacter pylori urease subunit A. J. Microbiol. 2015, 53, 406–414. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grahl, M.V.C.; Uberti, A.F.; Broll, V.; Bacaicoa-Caruso, P.; Meirelles, E.F.; Carlini, C.R. Proteus mirabilis Urease: Unsuspected Non-Enzymatic Properties Relevant to Pathogenicity. Int. J. Mol. Sci. 2021, 22, 7205. https://doi.org/10.3390/ijms22137205
Grahl MVC, Uberti AF, Broll V, Bacaicoa-Caruso P, Meirelles EF, Carlini CR. Proteus mirabilis Urease: Unsuspected Non-Enzymatic Properties Relevant to Pathogenicity. International Journal of Molecular Sciences. 2021; 22(13):7205. https://doi.org/10.3390/ijms22137205
Chicago/Turabian StyleGrahl, Matheus V. C., Augusto F. Uberti, Valquiria Broll, Paula Bacaicoa-Caruso, Evelin F. Meirelles, and Celia R. Carlini. 2021. "Proteus mirabilis Urease: Unsuspected Non-Enzymatic Properties Relevant to Pathogenicity" International Journal of Molecular Sciences 22, no. 13: 7205. https://doi.org/10.3390/ijms22137205
APA StyleGrahl, M. V. C., Uberti, A. F., Broll, V., Bacaicoa-Caruso, P., Meirelles, E. F., & Carlini, C. R. (2021). Proteus mirabilis Urease: Unsuspected Non-Enzymatic Properties Relevant to Pathogenicity. International Journal of Molecular Sciences, 22(13), 7205. https://doi.org/10.3390/ijms22137205