The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis
Abstract
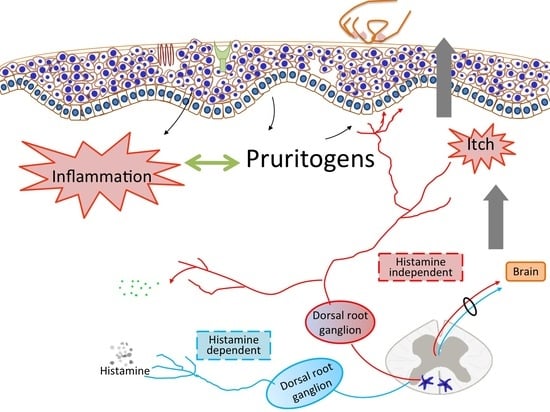
:1. Introduction
2. Histamine-Dependent Pruritogens
2.1. Histamine
2.2. Platelet-Activating Factor (PAF)
3. Histamine-Independent Pruritogens
3.1. Protease and Protease-Activated Receptors (PARs)
3.2. Thymic Stromal Lymphopoietin (TSLP) and TSLP Receptor (TSLPR)
3.3. IL-33
3.4. IL-4 and IL-13
3.5. IL-31
3.6. IL-6
3.7. Endothelin-1 (ET-1)
3.8. Neurotrophins (NTs)
3.9. Neuropeptides
3.10. Toll-Like Receptors (TLRs)
4. TCS and Topical Calcineurin Inhibitors (TCI) in Pruritus Control
5. MCs as a Therapeutic Target in AD and AD-Related Pruritus
6. Treatment Strategy for AD Patients with Concomitant Chronic Renal Insufficiency or Chronic Cholestatic Diseases
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahlgren, C.F. Itch and atopic dermatitis: An overview. J. Derm. 1999, 26, 770–779. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. J. Derm. Sci. 2013, 70, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Dong, X. Itch mechanisms and circuits. Annu. Rev. Biophys. 2014, 43, 331–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, F.E.; Simons, K.J. Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol. 2011, 128, 1139–1150.e1134. [Google Scholar] [CrossRef] [PubMed]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228.e213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Song, M.; Kikuchi, H.; Hisamichi, K.; Xu, X.L.; Greenspan, A.; Kato, M.; Chiou, C.F.; Kato, T.; Guzzo, C.; et al. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J. Derm. 2015, 42, 129–139. [Google Scholar] [CrossRef]
- Werfel, T.; Layton, G.; Yeadon, M.; Whitlock, L.; Osterloh, I.; Jimenez, P.; Liu, W.; Lynch, V.; Asher, A.; Tsianakas, A.; et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 1830–1837. [Google Scholar] [CrossRef]
- Hide, M.; Suzuki, T.; Tanaka, A.; Aoki, H. Long-term safety and efficacy of rupatadine in Japanese patients with itching due to chronic spontaneous urticaria, dermatitis, or pruritus: A 12-month, multicenter, open-label clinical trial. J. Derm. Sci. 2019, 94, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; Merwe, R.V.D. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Derm. 2019, 80, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of Etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, 2945. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Derm. 2020, 184, 437–449. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Derm. 2021, 184, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and safety of Lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis. JAMA Derm. 2020, 156, 411. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, J.I.; Pinter, A.; Pulka, G.; Poulin, Y.; Bouaziz, J.-D.; Wollenberg, A.; Murrell, D.F.; Alexis, A.; Lindsey, L.; Ahmad, F.; et al. Phase 2b randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020, 145, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Welsh, S.E.; Xiao, C.; Kaden, A.R.; Brzezynski, J.L.; Mohrman, M.A.; Wang, J.; Smieszek, S.P.; Przychodzen, B.; Ständer, S.; Polymeropoulos, C.; et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: Results from EPIONE, a randomized clinical trial. J. Eur. Acad. Derm. Venereol. 2020, 35, e338–e340. [Google Scholar]
- Rossbach, K.; Nassenstein, C.; Gschwandtner, M.; Schnell, D.; Sander, K.; Seifert, R.; Stark, H.; Kietzmann, M.; Bäumer, W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 2011, 190, 89–102. [Google Scholar] [CrossRef]
- Ohtsu, H.; Seike, M. Histamine and histamine receptors in allergic dermatitis. Handb. Exp. Pharm. 2016, 241, 333–345. [Google Scholar]
- Takahashi, A.; Tani, S.; Murota, H.; Katayama, I. Histamine modulates sweating and affects clinical manifestations of atopic dermatitis. Curr. Probl. Derm. 2016, 51, 50–56. [Google Scholar]
- Gutzmer, R.; Mommert, S.; Gschwandtner, M.; Zwingmann, K.; Stark, H.; Werfel, T. The histamine H4 receptor is functionally expressed on Th2 cells. J. Allergy Clin. Immunol. 2009, 123, 619–625. [Google Scholar] [CrossRef]
- Glatzer, F.; Gschwandtner, M.; Ehling, S.; Rossbach, K.; Janik, K.; Klos, A.; Bäumer, W.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Histamine induces proliferation in keratinocytes from patients with atopic dermatitis through the histamine 4 receptor. J. Allergy Clin. Immunol. 2013, 132, 1358–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, W.M.; Shenton, F.C.; Lethbridge, N.; Leurs, R.; Waldvogel, H.J.; Faull, R.L.M.; Lees, G.; Chazot, P.L. The histamine H4 receptor is functionally expressed on neurons in the mammalian cns. Br. J. Pharm. 2009, 157, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossbach, K.; Schaper, K.; Kloth, C.; Gutzmer, R.; Werfel, T.; Kietzmann, M.; Bäumer, W. Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy 2016, 71, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Schaper, K.; Rossbach, K.; Köther, B.; Stark, H.; Kietzmann, M.; Werfel, T.; Gutzmer, R. Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharm. Res. 2016, 113, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Mommert, S.; Hüer, M.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T. Histamine up-regulates oncostatin M expression in human M1 macrophages. Br. J. Pharm. 2020, 177, 600–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowden, J.M.; Zhang, M.; Dunford, P.J.; Thurmond, R.L. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J. Investig. Derm. 2010, 130, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Köchling, H.; Schaper, K.; Wilzopolski, J.; Gutzmer, R.; Werfel, T.; Bäumer, W.; Kietzmann, M.R.; Rossbach, K. Combined treatment with H1 and H4 receptor antagonists reduces inflammation in a mouse model of atopic dermatitis. J. Derm. Sci. 2017, 87, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, A.; Honda, T.; Doi, H.; Miyachi, Y.; Kabashima, K. An H1-histamine receptor antagonist decreases serum Interleukin-31 levels in patients with atopic dermatitis. Br. J. Derm. 2011, 164, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Kollmeier, A.; Franke, K.; Chen, B.; Dunford, P.J.; Greespan, A.J.; Xia, Y.; Xu, X.L.; Zhou, B.; Thurmond, R.L. The histamine H4 receptor antagonist, JNH 39758979, is effective in reducing histamine-induced pruritus in a randomized clinical study in healthy subjects. J. Pharm. Exp. 2014, 35, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Schauberger, E.; Peinhaupt, M.; Cazares, T.; Lindsley, A.W. Lipid mediators of allergic disease: Pathways, treatments, and emerging therapeutic targets. Curr. Allergy Asthma Rep. 2016, 16, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyer, K.D.; Percopo, C.M.; Xie, Z.; Yang, Z.; Kim, J.D.; Davoine, F.; Lacy, P.; Druey, K.M.; Moqbel, R.; Rosenberg, H.F. Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor–independent mechanism: Evidence for a novel receptor. J. Immunol. 2010, 184, 6327–6334. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.J.; Church, M.K.; Skov, P.S. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. J. Allergy Clin. Immunol. 1997, 99, 640–647. [Google Scholar] [CrossRef]
- Lee, C.-H. Progress of pruritus research in atopic dermatitis. Biomol. Ther. 2010, 18, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Ocana, J.A.; Romer, E.; Sahu, R.; Pawelzik, S.-C.; FitzGerald, G.A.; Kaplan, M.H.; Travers, J.B. Platelet-activating factor–induced reduction in contact hypersensitivity responses is mediated by mast cells via cyclooxygenase-2–dependent mechanisms. J. Immunol. 2018, 200, 4004–4011. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Jeong, S.K.; Lee, S.H. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med. J. 2010, 51, 808. [Google Scholar] [CrossRef] [Green Version]
- Moniaga, C.S.; Jeong, S.K.; Egawa, G.; Nakajima, S.; Hara-Chikuma, M.; Jeon, J.E.; Lee, S.H.; Hibino, T.; Miyachi, Y.; Kabashima, K. Protease activity enhances production of thymic stromal lymphopoietin and basophil accumulation in flaky tail mice. Am. J. Pathol. 2013, 182, 841–851. [Google Scholar] [CrossRef]
- Ramachandran, R.; Hollenberg, M.D. Proteinases and signalling: Pathophysiological and therapeutic implications via PARs and more. Br. J. Pharm. 2008, 153, S263–S282. [Google Scholar] [CrossRef]
- Nomura, H.; Suganuma, M.; Takeichi, T.; Kono, M.; Isokane, Y.; Sunagawa, K.; Kobashi, M.; Sugihara, S.; Kajita, A.; Miyake, T.; et al. Multifaceted analyses of epidermal serine protease activity in patients with atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 913. [Google Scholar] [CrossRef] [Green Version]
- Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef]
- Briot, A.; Deraison, C.; Lacroix, M.; Bonnart, C.; Robin, A.; Besson, C.; Dubus, P.; Hovnanian, A. Kallikrein 5 induces atopic dermatitis–like lesions through PAR2-mediated thymic stromal lymphopoietin expression in netherton syndrome. J. Exp. Med. 2009, 206, 1135–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.J.; Mack, M.R.; Oetjen, L.K.; Trier, A.M.; Council, M.L.; Pavel, A.B.; Guttman-Yassky, E.; Kim, B.S.; Liu, Q. Kallikrein 7 promotes atopic dermatitis-associated itch independently of skin inflammation. J. Investig. Derm. 2020, 140, 1244–1252.e1244. [Google Scholar] [CrossRef]
- Zhu, Y.; Underwood, J.; Macmillan, D.; Shariff, L.; O’Shaughnessy, R.; Harper, J.I.; Pickard, C.; Friedmann, P.S.; Healy, E.; Di, W.-L. Persistent kallikrein 5 activation induces atopic dermatitis-like skin architecture independent of PAR2 activity. J. Allergy Clin. Immunol. 2017, 140, 1310–1322.e1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Munanairi, A.; Liu, X.-Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S.; et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J. Investig. Derm. 2020, 140, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Buhl, T.; Ikoma, A.; Kempkes, C.; Cevikbas, F.; Sulk, M.; Buddenkotte, J.; Akiyama, T.; Crumrine, D.; Camerer, E.; Carstens, E.; et al. Protease-activated receptor-2 regulates neuro-epidermal communication in atopic dermatitis. Front. Immunol. 2020, 11, 1740. [Google Scholar] [CrossRef]
- Braz, J.M.; Dembo, T.; Charruyer, A.; Ghadially, R.; Fassett, M.S.; Basbaum, A.I. Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness. Proc. Natl. Acad. Sci. USA 2021, 118, e2021386118. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Carstens, E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: Potential role in itch. J. Neurophysiol. 2009, 102, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef]
- Chung, K.; Pitcher, T.; Grant, A.D.; Hewitt, E.; Lindstrom, E.; Malcangio, M. Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour. Neurobiol. Pain. 2019, 6, 100032. [Google Scholar] [CrossRef]
- Barr, T.P.; Garzia, C.; Guha, S.; Fletcher, E.K.; Nguyen, N.; Wieschhaus, A.J.; Ferrer, L.; Covic, L.; Kuliopulos, A. PAR2 pepducin-based suppression of inflammation and itch in atopic dermatitis models. J. Investig. Derm. 2019, 139, 412–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andoh, T.; Kuraishi, Y. Antipruritic mechanisms of topical E6005, a phosphodiesterase 4 inhibitor: Inhibition of responses to proteinase-activated receptor 2 stimulation mediated by increase in intracellular cyclic AMP. J. Derm. Sci. 2014, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, M.; Hirota, T.; Jodo, A.I.; Doi, S.; Kameda, M.; Fujita, K.; Miyatake, A.; Enomoto, T.; Noguchi, E.; Yoshihara, S.; et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2009, 40, 368–374. [Google Scholar] [CrossRef]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Derm. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef]
- Dong, H.; Hu, Y.; Liu, L.; Zou, M.; Huang, C.; Luo, L.; Yu, C.; Wan, X.; Zhao, H.; Chen, J.; et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci. Rep. 2016, 6, 39559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerkan, L.; Sonesson, A.; Schenck, K. Multiple functions of the new cytokine-based antimicrobial peptide thymic stromal lymphopoietin (TSLP). Pharmaceuticals 2016, 9, 41. [Google Scholar] [CrossRef]
- Lee, E.B.; Kim, K.W.; Hong, J.Y.; Jee, H.M.; Sohn, M.H.; Kim, K.E. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr. Allergy Immunol. 2010, 21, e457–e460. [Google Scholar] [CrossRef] [PubMed]
- Takai, T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Hanabuchi, S.; Soumelis, V.; Yuan, W.; Ho, S.; de Waal Malefyt, R.; Liu, Y.-J. Human thymic stromal lymphopoietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat. Immunol. 2004, 5, 426–434. [Google Scholar] [CrossRef]
- Pattarini, L.; Trichot, C.; Bogiatzi, S.; Grandclaudon, M.; Meller, S.; Keuylian, Z.; Durand, M.; Volpe, E.; Madonna, S.; Cavani, A.; et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017, 214, 1529–1546. [Google Scholar] [CrossRef] [Green Version]
- Marschall, P.; Wei, R.; Segaud, J.; Yao, W.; Hener, P.; German, B.F.; Meyer, P.; Hugel, C.; Ada Da Silva, G.; Braun, R.; et al. Dual function of Langerhans cells in skin TSLP-promoted TFH differentiation in mouse atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, K.; Fujiyama, T.; Yamaguchi, H.; Waki, M.; Tokura, Y. TSLP directly interacts with skin-homing Th2 cells highly expressing its receptor to enhance IL-4 production in atopic dermatitis. J. Investig. Derm. 2015, 135, 3017–3024. [Google Scholar] [CrossRef] [Green Version]
- Rochman, Y.; Dienger-Stambaugh, K.; Richgels, P.K.; Lewkowich, I.P.; Kartashov, A.V.; Barski, A.; Khurana Hershey, G.K.; Leonard, W.J.; Singh, H. TSLP signaling in CD4+T cells programs a pathogenic T helper 2 cell state. Sci. Signal. 2018, 11, eaam8858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochiai, S.; Jagot, F.; Kyle, R.L.; Hyde, E.; White, R.F.; Prout, M.; Schmidt, A.J.; Yamane, H.; Lamiable, O.; Le Gros, G.; et al. Thymic stromal lymphopoietin drives the development of IL-13+ Th2 cells. Proc. Natl. Acad. Sci. USA 2018, 115, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Wallmeyer, L.; Dietert, K.; Sochorová, M.; Gruber, A.D.; Kleuser, B.; Vávrová, K.; Hedtrich, S. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci. Rep. 2017, 7, 774. [Google Scholar] [CrossRef] [Green Version]
- Salter, B.M.; Oliveria, J.P.; Nusca, G.; Smith, S.G.; Watson, R.M.; Comeau, M.; Sehmi, R.; Gauvreau, G.M. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J. Allergy Clin. Immunol. 2015, 136, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Salabert-Le Guen, N.; Hémont, C.; Delbove, A.; Poli, C.; Braudeau, C.; Fantou, A.; Amouriaux, K.; Bériou, G.; Martin, J.C.; Colas, L.; et al. Thymic stromal lymphopoietin does not activate human basophils. J. Allergy Clin. Immunol. 2018, 141, 1476–1479.e6. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Bae, H.C.; Ko, N.Y.; Lee, S.H.; Jeong, S.H.; Lee, H.; Ryu, W.-I.; Kye, Y.C.; Son, S.W. Thymic stromal lymphopoietin downregulates filaggrin expression by signal transducer and activator of transcription 3 (STAT3) and extracellular signal-regulated kinase (ERK) phosphorylation in keratinocytes. J. Allergy Clin. Immunol. 2015, 136, 205–208.e9. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J. Investig. Derm. 2013, 133, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Unutmaz, D.; Moussion, C.; Ortega, N.; Girard, J.-P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: A novel ‘alarmin’? PLoS ONE 2008, 3, e3331. [Google Scholar]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Derm. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, N.T.; Martin, M.U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 2016, 17, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Derm. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Kindi, A.A.; Williams, H.; Matsuda, K.; Alkahtani, A.M.; Saville, C.; Bennett, H.; Alshammari, Y.; Tan, S.Y.; O’Neill, C.; Tanaka, A.; et al. Staphylococcus aureus second immunoglobulin-binding protein drives atopic dermatitis via IL-33. J. Allergy Clin. Immunol. 2021, 147, 1354–1368.e3. [Google Scholar] [CrossRef]
- Tamagawa-Mineoka, R.; Okuzawa, Y.; Masuda, K.; Katoh, N. Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Derm. 2014, 70, 882–888. [Google Scholar] [CrossRef]
- Nakamura, N.; Tamagawa-Mineoka, R.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum corneum interleukin-33 expressions correlate with the degree of lichenification and pruritus in atopic dermatitis lesions. Clin. Immunol. 2019, 201, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Wang, Z.; Zuberbier, T.; Babina, M. Cytokines stimulated by IL-33 in human skin mast cells: Involvement of NF-κB and p38 at distinct levels and potent co-operation with FcεRI and MRGPRX2. Int. J. Mol. Sci. 2021, 22, 3580. [Google Scholar] [CrossRef]
- Imai, Y.; Yasuda, K.; Nagai, M.; Kusakabe, M.; Kubo, M.; Nakanishi, K.; Yamanishi, K. IL-33–induced atopic dermatitis–like inflammation in mice is mediated by group 2 innate lymphoid cells in concert with basophils. J. Investig. Derm. 2019, 139, 2185–2194.e3. [Google Scholar] [CrossRef]
- Murakami-Satsutani, N.; Ito, T.; Nakanishi, T.; Inagaki, N.; Tanaka, A.; Vien, P.T.X.; Kibata, K.; Inaba, M.; Nomura, S. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol. Int. 2014, 63, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, W.-I.; Lee, H.; Bae, H.C.; Ryu, H.J.; Son, S.W. IL-33 down-regulates filaggrin expression by inducing STAT3 and ERK phosphorylation in human keratinocytes. J. Derm. Sci. 2016, 82, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Hu, X.; Yang, W.; Yasheng, H.; Liu, S.; Zhang, W.; Zhou, Y.; Cui, W.; Zhu, J.; Qiao, Z.; et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019, 67, 1680–1693. [Google Scholar] [CrossRef]
- Howell, M.D.; Kim, B.E.; Gao, P.; Grant, A.V.; Boguniewicz, M.; DeBenedetto, A.; Schneider, L.; Beck, L.A.; Barnes, K.C.; Leung, D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2009, 124, R7–R12. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.M.; Heller, N.M. Commentary: IL-4 and IL-13 receptors and signaling. Cytokine 2015, 75, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Chiricozzi, A.; Maurelli, M.; Peris, K.; Girolomoni, G. Targeting IL-4 for the treatment of atopic dermatitis. Immunotargets Ther. 2020, 9, 151–156. [Google Scholar] [CrossRef]
- Bao, K.; Reinhardt, R.L. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine 2015, 75, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Furue, K.; Ito, T.; Tsuji, G.; Ulzii, D.; Vu, Y.H.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. The IL-13-OVOL1–FLG axis in atopic dermatitis. Immunology 2019, 158, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miake, S.; Tsuji, G.; Takemura, M.; Hashimoto-Hachiya, A.; Vu, Y.H.; Furue, M.; Nakahara, T. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced CCL 17 and CCL 22 production in dendritic cells: Implications for atopic dermatitis. Int. J. Mol. Sci. 2019, 20, 4053. [Google Scholar] [CrossRef] [Green Version]
- Zheng, T.; Oh, M.H.; Oh, S.Y.; Schroeder, J.T.; Glick, A.B.; Zhu, Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J. Investig. Derm. 2009, 129, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Bitton, A.; Avlas, S.; Reichman, H.; Itan, M.; Karo-Atar, D.; Azouz, N.P.; Rozenberg, P.; Diesendruck, Y.; Nahary, L.; Rothenberg, M.E.; et al. A key role for IL-13 signaling via the type 2 IL-4 receptor in experimental atopic dermatitis. Sci. Immunol. 2020, 5, eaaw2938. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Hong, H.C.-H.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Derm. 2018, 78, S28–S36. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef]
- Nakashima, C.; Otsuka, A.; Kabashima, K. Interleukin-31 and interleukin-31 receptor: New therapeutic targets for atopic dermatitis. Exp. Derm. 2018, 27, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasraie, S.; Niebuhr, M.; Werfel, T. Interleukin (IL)-31 induces pro-inflammatory cytokines in human monocytes and macrophages following stimulation with staphylococcal exotoxins. Allergy 2009, 65, 712–721. [Google Scholar] [CrossRef]
- Stott, B.; Lavender, P.; Lehmann, S.; Pennino, D.; Durham, S.; Schmidt-Weber, C.B. Human IL-31 is induced by IL-4 and promotes Th2-driven inflammation. J. Allergy Clin. Immunol. 2013, 132, 446. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Kato, A.; Fujii, E.; Watanabe, T.; Takashima, Y.; Matsushita, H.; Furuhashi, T.; Morita, A. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J. Derm. Sci 2014, 74, 229–235. [Google Scholar] [CrossRef]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.C.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef]
- Raap, U.; Wichmann, K.; Bruder, M.; Stander, S.; Wedi, B.; Kapp, A.; Werfel, T. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008, 122, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Kunsleben, N.; Rüdrich, U.; Gehring, M.; Novak, N.; Kapp, A.; Raap, U. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J. Investig. Derm. 2015, 135, 1908–1911. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, J.; Wong, C.-K.; Leung, K.M.-L.; Qiu, H.-N.; Chow, J.Y.-S.; Choi, A.O.K.; Lam, C.W.-K. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: Implications in atopic dermatitis. PLoS ONE 2012, 7, e29815. [Google Scholar]
- Raap, U.; Gehring, M.; Kleiner, S.; Rüdrich, U.; Eiz-Vesper, B.; Haas, H.; Kapp, A.; Gibbs, B.F. Human basophils are a source of—and are differentially activated by—IL-31. Clin. Exp. Allergy 2017, 47, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, C.; Marquardt, Y.; Czaja, K.; Wenzel, J.; Frank, J.; Lüscher-Firzlaff, J.; Lüscher, B.; Baron, J.M. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 2012, 129, 426–433.e428. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.H.; Pfaff, C.M.; Cornelissen, C.; Amann, P.M.; Marquardt, Y.; Czaja, K.; Kim, A.; Lüscher, B.; Baron, J.M. Control of the physical and antimicrobial skin barrier by an IL-31–IL-1 signaling network. J. Immunol. 2016, 196, 3233–3244. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Singh, B.; Jegga, A.G.; Shanmukhappa, K.S.; Edukulla, R.; Khurana, G.H.; Medvedovic, M.; Dillon, S.R.; Madala, S.K. IL-31-driven skin remodeling involves epidermal cell proliferation and thickening that lead to impaired skin-barrier function. PLoS ONE 2016, 11, e0161877. [Google Scholar]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and Th 2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [Green Version]
- Hawro, T.; Saluja, R.; Weller, K.; Altrichter, S.; Metz, M.; Maurer, M. Interleukin-31 does not induce immediate itch in atopic dermatitis patients and healthy controls after skin challenge. Allergy 2014, 69, 113–117. [Google Scholar] [CrossRef]
- Meng, J.; Moriyama, M.; Feld, M.; Buddenkotte, J.; Buhl, T.; Szöllösi, A.; Zhang, J.; Miller, P.; Ghetti, A.; Fischer, M.; et al. New mechanism underlying IL-31–induced atopic dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1677–1689.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, C.; Gandhi, R.; Botelho, F.; Ho, L.; Paolini, J. Oncostatin M induction of monocyte chemoattractant protein 1 is inhibited by anti-oncostatin M receptor beta monoclonal antibody KPL-716. Acta Derm. Venereol. 2020, 100, adv00197. [Google Scholar] [CrossRef]
- Rincón, M.A.J.; Nakamura, T.; Fikrig, E.; Flavell, R.A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J. Exp. Med. 1997, 185, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toshitani, A.; Ansel, J.C.; Chan, S.C.; Li, S.H.; Hanifin, J.M. Increased interleukin 6 production by T cells derived from patients with atopic dermatitis. J. Investig. Derm. 1993, 100, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, P.; Kempuraj, D.; Gioacchino, M.D.; Boucher, W.; Letourneau, R.; Kandere, K.; Barbacane, R.C.; Reale, M.; Felaco, M.; Frydas, S.; et al. Interleukin-6 and mast cells. Allergy Asthma Proc. 2002, 23, 331–335. [Google Scholar]
- Fedenko, E.S.; Elisyutina, O.G.; Filimonova, T.M.; Boldyreva, M.N.; Burmenskaya, O.V.; Rebrova, O.Y.; Yarilin, A.A.; Khaitov, R.M. Cytokine gene expression in the skin and peripheral blood of atopic dermatitis patients and healthy individuals. Self/Nonself 2011, 2, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Navarini, A.A.; French, L.E.; Hofbauer, G.F.L. Interrupting IL-6–receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J. Allergy Clin. Immunol. 2011, 128, 1128–1130. [Google Scholar] [CrossRef]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. IL-6/p-BTK/P-ERK signaling mediates calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef] [Green Version]
- Konda, D.; Chandrashekar, L.; Rajappa, M.; Kattimani, S.; Thappa, D.M.; Ananthanarayanan, P.H. Serotonin and interleukin-6: Association with pruritus severity, sleep quality and depression severity in prurigo nodularis. Asian J. Psychiatr. 2015, 17, 24–28. [Google Scholar] [CrossRef]
- Kido-Nakahara, M.; Buddenkotte, J.; Kempkes, C.; Ikoma, A.; Cevikbas, F.; Akiyama, T.; Nunes, F.; Seeliger, S.; Hasdemir, B.; Mess, C.; et al. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1-induced pruritus. J. Clin. Investig. 2014, 124, 2683–2695. [Google Scholar] [CrossRef] [Green Version]
- Aktar, M.K.; Kido-Nakahara, M.; Furue, M.; Nakahara, T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy 2015, 70, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Tsybikov, N.N.; Petrisheva, I.V.; Kuznik, B.I.; Magen, E. Plasma endothelin-1 levels during exacerbation of atopic dermatitis. Allergy Asthma Proc. 2015, 36, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.S.; Yen, Y.T.; Lin, S.H.; Lee, C.H. IL-17A induces endothelin-1 expression through p38 pathway in prurigo nodularis. J. Investig. Derm. 2020, 140, 702–706.e2. [Google Scholar] [CrossRef]
- Borowczyk, J.; Shutova, M.; Brembilla, N.C.; Boehncke, W.-H. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J. Allergy Clin. Immunol. 2021. [Google Scholar] [CrossRef]
- Yamada, Y.; Matsumoto, T. House dust mites induce production of endothelin-1 and matrix metalloproteinase-9 in keratinocytes via proteinase-activated receptor-2 activation. Int. Arch. Allergy Immunol. 2017, 173, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kido-Nakahara, M.; Wang, B.; Ohno, F.; Tsuji, G.; Ulzii, D.; Takemura, M.; Furue, M.; Nakahara, T. Inhibition of mite-induced dermatitis, pruritus, and nerve sprouting in mice by the endothelin receptor antagonist bosentan. Allergy 2020, 76, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Nockher, W.A.; Renz, H. Neurotrophins in allergic diseases: From neuronal growth factors to intercellular signaling molecules. J. Allergy Clin. Immunol. 2006, 117, 583–589. [Google Scholar] [CrossRef]
- Dou, Y.C.; Hagströmer, L.; Emtestam, L.; Johansson, O. Increased nerve growth factor and its receptors in atopic dermatitis: An immunohistochemical study. Arch. Derm. 2006, 298, 31–37. [Google Scholar] [CrossRef]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Derm. 2002, 147, 71–79. [Google Scholar] [CrossRef]
- Papoiu, A.D.P.; Wang, H.; Nattkemper, L.; Tey, H.L.; Ishiuji, Y.; Chan, Y.H.; Schmelz, M.; Yosipovitch, G. A study of serum concentrations and dermal levels of NGF in atopic dermatitis and healthy subjects. Neuropeptides 2011, 45, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Herbrüggen, O.; Fölster-Holst, R.; Von Elstermann, M.; Augustin, M.; Hellweg, R. Clinical relevance of nerve growth factor serum levels in patients with atopic dermatitis and psoriasis. Int. Arch. Allergy Immunol. 2007, 144, 211–216. [Google Scholar] [CrossRef]
- Roggenkamp, D.; Falkner, S.; Stäb, F.; Petersen, M.; Schmelz, M.; Neufang, G. Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J. Investig. Derm. 2012, 132, 1892–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritas, S.K.; Caraffa, A.; Antinolfi, P.; Saggini, A.P.A.; Rosati, M.; Tei, M.; Speziali, A.; Saggini, R.; Pandolfi, F.; Pantalone, A.; et al. Nerve growth factor interactions with mast cells. Int. J. Immunopathol. Pharm. 2014, 21, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [Green Version]
- Fölster-Holst, R.; Papakonstantinou, E.; Rüdrich, U.; Buchner, M.; Pite, H.; Gehring, M.; Kapp, A.; Weidinger, S.; Raap, U. Childhood atopic dermatitis-brain-derived neurotrophic factor correlates with serum eosinophil cationic protein and disease severity. Allergy 2016, 71, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Raap, U.; Goltz, C.; Deneka, N.; Bruder, M.; Renz, H.; Kapp, A.; Wedi, B. Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J. Allergy Clin. Immunol. 2005, 115, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Guseva, D.; Rüdrich, U.; Kotnik, N.; Gehring, M.; Patsinakidis, N.; Agelopoulos, K.; Ständer, S.; Homey, B.; Kapp, A.; Gibbs, B.F.; et al. Neuronal branching of sensory neurons is associated with BDNF-positive eosinophils in atopic dermatitis. Clin. Exp. Allergy 2020, 50, 577–584. [Google Scholar] [CrossRef]
- Roblin, D.; Yosipovitch, G.; Boyce, B.; Robinson, J.; Sandy, J.; Mainero, V.; Wickramasinghe, R.; Anand, U.; Anand, P. Topical TrkA kinase inhibitor CT327 is an effective, novel therapy for the treatment of pruritus due to psoriasis: Results from experimental studies, and efficacy and safety of CT327 in a phase 2b clinical trial in patients with psoriasis. Acta Derm. Venereol. 2015, 95, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide substance P and the immune response. Cell. Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef] [Green Version]
- Meixiong, J.; Dong, X.Z. Mas-related g protein-coupled receptors and the biology of itch sensation. Annu. Rev. Genet. 2017, 51, 105–121. [Google Scholar] [CrossRef]
- Lönndahl, L.; Rasul, A.; Lonne-Rahm, S.-B.; Holst, M.; Johansson, B.; El-Nour, H.; Radu Djurfeldt, D.; Nordlind, K. Tachykinin upregulation in atopic dermatitis. Immunopharmacol. Immunotoxicol. 2019, 41, 117–122. [Google Scholar] [CrossRef]
- Paramita, D.A.; Nasution, K.; Lubis, N.Z. Relationship of substance P with the degree of atopic dermatitis severity. Clin. Cosmet. Investig. Derm. 2021, 14, 551–555. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front. Cell. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related g protein–coupled receptor X2 on mast cell–mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [Green Version]
- Taracanova, A.; Tsilioni, I.; Conti, P.; Norwitz, E.R.; Leeman, S.E.; Theoharides, T.C. Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. USA 2018, 115, E9381–E9390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taracanova, A.; Alevizos, M.; Karagkouni, A.; Weng, Z.; Norwitz, E.; Conti, P.; Leeman, S.E.; Theoharides, T.C. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. USA 2017, 114, E4002–E4009. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wang, L.; Clark, J.D.; Kingery, W.S. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul. Pept. 2013, 186, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Andoh, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J. Pharmacol. Exp. Ther. 1998, 286, 1140–1145. [Google Scholar] [PubMed]
- Azimi, E.; Reddy, V.B.; Shade, K.-T.C.; Anthony, R.M.; Talbot, S.; Pereira, P.J.S.; Lerner, E.A. Dual action of neurokinin-1 antagonists on Mas-related GPCRS. JCI Insight 2016, 1, e89362. [Google Scholar] [CrossRef] [PubMed]
- Azimi, E.; Reddy, V.B.; Pereira, P.J.S.; Talbot, S.; Woolf, C.J.; Lerner, E.A. Substance P activates Mas-related G protein–coupled receptors to induce itch. J. Allergy Clin. Immunol. 2017, 140, 447–453.e3. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, T.; Tominaga, M.; Davoodi, A.; Nagamine, M.; Blansit, K.; Horwitz, A.; Carstens, M.I.; Carstens, E. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J. Neurophysiol. 2013, 109, 742–748. [Google Scholar] [CrossRef] [Green Version]
- Sheahan, T.D.; Warwick, C.A.; Fanien, L.G.; Ross, S.E. The neurokinin-1 receptor is expressed with gastrin-releasing peptide receptor in spinal interneurons and modulates itch. J. Neurosci. 2020, 40, 8816–8830. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-P.; Cnaan, A.; Zhao, H.; Douglas, S.D. Detection of full-length and truncated neurokinin-1 receptor mRNA expression in human brain regions. J. Neurosci. Methods 2008, 168, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmura, T.; Hayashi, T.; Satoh, Y.; Konomi, A.; Jung, B.; Satoh, H. Involvement of substance P in scratching behaviour in an atopic dermatitis model. Eur. J. Pharm. 2004, 491, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Stander, S.; Siepmann, D.; Herrgott, I.; Sunderkotter, C.; Luger, T.A. Targeting the neurokinin receptor 1 with aprepitant: A novel antipruritic strategy. PLoS ONE 2010, 5, e10968. [Google Scholar] [CrossRef] [PubMed]
- Lönndahl, L.; Holst, M.; Bradley, M.; Killasli, H.; Heilborn, J.; Hall, M.; Theodorsson, E.; Holmberg, J.; Nordlind, K. Substance P antagonist aprepitant shows no additive effect compared with standardized topical treatment alone in patients with atopic dermatitis. Acta. Derm. Venereol. 2018, 98, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Granstein, R.D.; Wagner, J.A.; Stohl, L.L.; Ding, W. Calcitonin gene-related peptide: Key regulator of cutaneous immunity. Acta Physiol. 2015, 213, 586–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.H.; Stohl, L.L.; Wagner, J.A.; Granstein, R.D. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J. Immunol. 2008, 181, 6020–6026. [Google Scholar] [CrossRef] [Green Version]
- Järvikallio, A.; Harvima, I.T.; Naukkarinen, A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch. Derm. Res. 2003, 295, 2–7. [Google Scholar] [CrossRef]
- Hodeib, A.; EI-Samad, Z.A.; Hanafy, H.; EI-Latief, A.A.; EI-Bendary, A.; Abu-Raya, A. Nerve growth factor, neuropeptides and cutaneous nerves in atopic dermatitis. Indian J. Derm. 2010, 55, 135–139. [Google Scholar]
- McCoy, E.S.; Taylor-Blake, B.; Sarah, S.E.; Pribisko, A.L.; Zheng, J.; Zylka, M.J. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 2013, 78, 138–151. [Google Scholar] [CrossRef] [Green Version]
- Katsuno, M.; Aihara, M.; Kojima, M.; Osuna, H.; Hosoi, J.; Nakamura, M.; Toyoda, M.; Matsuda, H.; Ikezawa, Z. Neuropeptides concentrations in the skin of a murine (NC/Nga mice) model of atopic dermatitis. J. Derm. Sci. 2003, 33, 55–65. [Google Scholar] [CrossRef]
- Andoh, T.; Asakawa, Y.; Kuraishi, Y. Non-myelinated C-fibers, but not myelinated α-fibers, elongate into the epidermis in dry skin with itch. Neurosci. Lett. 2018, 672, 84–89. [Google Scholar] [CrossRef]
- Umemoto, N.; Kakurai, M.; Okazaki, H.; Kiyosawa, T.; Demitsu, T.; Nakagawa, H. Serum levels of vasoactive intestinal peptide are elevated in patients with atopic dermatitis. J. Derm. Sci. 2003, 31, 161–164. [Google Scholar] [CrossRef]
- Teresiak-Mikołajczak, E.; Czarnecka-Operacz, M.; Jenerowicz, D.; Silny, W. Neurogenic markers of the inflammatory process in atopic dermatitis: Relation to the severity and pruritus. Pestepy Derm. Alergol. 2013, 5, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ganea, D.; Hooper, K.M.; Kong, W. The neuropeptide vasoactive intestinal peptide: Direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015, 213, 442–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, N.; Miyahara, N.; Taniguchi, A.; Morichika, D.; Senoo, S.; Fujii, U.; Itano, J.; Gion, Y.; Kiura, K.; Kanehiro, A.; et al. Requirement for neuropeptide Y in the development of type 2 responses and allergen-induced airway hyperresponsiveness and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L407–L417. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the grp pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef] [Green Version]
- Tirado-Sánchez, A.; Bonifaz, A.; Ponce-Olivera, R.M. Serum gastrin-releasing peptide levels correlate with disease severity and pruritus in patients with atopic dermatitis. Br. J. Derm. 2015, 173, 298–300. [Google Scholar] [CrossRef]
- Pagani, M.; Albisetti, G.W.; Sivakumar, N.; Wildner, H.; Santello, M.; Johannssen, H.C.; Zeilhofer, H.U. How gastrin-releasing peptide opens the spinal gate for itch. Neuron 2019, 103, 102–117.e105. [Google Scholar] [CrossRef] [Green Version]
- Vasiadi, M.; Mondolfi, A.P.; Alysandratos, K.D.; Therianou, A.; Katsarou-Katsari, A.; Petrakopoulou, T.; Theoharidis, A.; Miniati, A.; Theoharides, T.C. Neurotensin serum levels and skin gene expression are increased in atopic dermatitis. Br. J. Derm. 2013, 169, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, L.; Emtestam, L.; Stridsberg, M.; Talme, T. Expression pattern of somatostatin receptor subtypes 1-5 in human skin: An immunohistochemical study of healthy subjects and patients with psoriasis or atopic dermatitis. Exp. Derm. 2006, 15, 950–957. [Google Scholar] [CrossRef]
- Sun, L.; Liu, W.; Zhang, L. The role of Toll-like receptors in skin host defense, psoriasis, and atopic dermatitis. J. Immunol. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.-C.; Feng, C.; Yan, M. Analysis of the association of polymorphisms rs5743708 in TLR2 and rs4986790 in TLR4 with atopic dermatitis risk. Immunol. Investig. 2018, 48, 169–180. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, D.; Cai, X.; Cui, D.; Fang, R.; Zhang, W.; Yu, B.; Wang, X. Enhancement of chemokine mrna expression by toll-like receptor 2 stimulation in human peripheral blood mononuclear cells of patients with atopic dermatitis. Biomed. Res. Int. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, Y.; Zhang, J.; Dou, X.; Yang, H.; Shao, Y.; Wang, K.; Yu, B.; Zhang, W.; Lau, H.Y.A. Impaired Toll-like receptor 2-mediated Th1 and Th17/22 cytokines secretion in human peripheral blood mononuclear cells from patients with atopic dermatitis. J. Transl. Med. 2015, 13, 384. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, N.; Tamagawa-Mineoka, R.; Ueta, M.; Konishi, E.; Yasuike, R.; Masuda, K.; Matsunaka, H.; Murakami, Y.; Yokosawa, E.; Katoh, N. Stratum corneum Toll-like receptor 3 expressions correlate with the severity of atopic dermatitis lesions. J. Derm. Sci. 2019, 94, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Szöllősi, A.G.; McDonald, I.; Szabó, I.L.; Meng, J.; van den Bogaard, E.; Steinhoff, M. TLR3 in chronic human itch: A keratinocyte-associated mechanism of peripheral itch sensitization. J. Investig. Derm. 2019, 139, 2393–2396.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuike, R.; Tamagawa-Mineoka, R.; Ueta, M.; Nakamura, N.; Kinoshita, S.; Katoh, N. The role of Toll-like receptor 3 in chronic contact hypersensitivity induced by repeated elicitation. J. Derm. Sci. 2017, 88, 184–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Berta, T.; Xu, Z.-Z.; Park, C.-K.; Zhang, L.; Lü, N.; Liu, Q.; Liu, Y.; Gao, Y.-J.; Liu, Y.-C.; et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Investig. 2012, 122, 2195–2207. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.Z.; Park, C.K.; Berta, T.; Ji, R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010, 13, 1460–1462. [Google Scholar] [CrossRef]
- Liu, T.; Han, Q.; Chen, G.; Huang, Y.; Zhao, L.-X.; Berta, T.; Gao, Y.-J.; Ji, R.-R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016, 157, 806–817. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Lee, S.; Tierney, J.A.; Elmquist, J.K.; Burton, M.D.; Gautron, L. TLR4 signaling selectively and directly promotes CGRP release from vagal afferents in the mouse. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Kim, D.; Lee, J.; Min, H.; Wall, E.; Lee, C.J.; Simon, M.I.; Lee, S.J.; Han, S.K. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 3371–3376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, A.; Guttman-Yassky, E. Topical agents for the treatment of atopic dermatitis. Expert Rev. Clin. Immunol. 2019, 15, 369–382. [Google Scholar] [CrossRef]
- Lehto, M.; Savinko, T.; Wolff, H.; Kvist, P.H.; Kemp, K.; Lauerma, A.; Alenius, H. A murine model of epicutaneous protein sensitization is useful to study efficacies of topical drugs in atopic dermatitis. Int. Immunopharmacol. 2010, 10, 377–384. [Google Scholar] [CrossRef]
- Nakahara, T.; Morimoto, H.; Murakami, N.; Furue, M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr. Allergy. Immunol. 2018, 29, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Paller, A.S.; Irvine, A.D.; Sidbury, R.; Eichenfield, L.F.; Werfel, T.; Bieber, T. Topical therapy of atopic dermatitis with a focus on pimecrolimus. J. Eur. Acad. Derm. Venereol. 2021. [Google Scholar] [CrossRef]
- Reber, L.L.; Frossard, N. Targeting mast cells in inflammatory diseases. Pharmacol. Ther. 2014, 142, 416–435. [Google Scholar] [CrossRef]
- Paivandy, A.; Pejler, G. Novel strategies to target mast cells in disease. J. Innate Immun. 2021, 13, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Siebenhaar, F.; Redegeld, F.A.; Bischoff, S.C.; Gibbs, B.F.; Maurer, M. Mast cells as drivers of disease and therapeutic targets. Trends Immunol. 2018, 39, 151–162. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Ryu, K.-J.; Kim, H.-M. Anticancer agent ABT-737 possesses anti-atopic dermatitis activity via blockade of caspase-1 in atopic dermatitis in vitro and in vivo models. Immunopharmacol. Immunotoxicol. 2018, 40, 319–326. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; García-Montero, A.; Mayado, A.; Caldas, C.T.C.; Muñoz-González, J.I.; Mollejo, M.; et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 kit mutations and review of the literature. Oncotarget 2017, 8, 68950–68963. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Sang, Y.; Yang, M.; Chen, X.; Tang, W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients. Medicine 2018, 97, e10633. [Google Scholar] [CrossRef]
- Martin, C.E.; Clotet-Freixas, S.; Farragher, J.F.; Hundemer, G.L. Have we just scratched the surface? A narrative review of uremic pruritus in 2020. Can. J. Kidney Health Dis. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, E.; Komenda, P.; Lerner, B.; Askin, N.; Bohm, C.; Shaw, J.; Tangri, N.; Rigatto, C. Treatment of uremic pruritus: A systematic review. Am. J. Kidney. Dis. 2017, 70, 638–655. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, J.I.; Brieva, J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019, 5, 339–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oweis, A.O.; Al-Qarqaz, F.; Bodoor, K.; Heis, L.; Alfaqih, M.A.; Almomani, R.; Obeidat, M.A.; Alshelleh, S.A. Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Cytokine 2021, 138, 155369. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Vasavda, C.; Ho, B.; Meixiong, J.; Dong, X.; Kwatra, S.G. Cholestatic pruritus: Emerging mechanisms and therapeutics. J. Am. Acad. Derm. 2019, 81, 1371–1378. [Google Scholar] [CrossRef] [PubMed]

| Target | Study Phase (Trial Identification) | Enrollment | Efficacy | Status | Ref. | |
|---|---|---|---|---|---|---|
| JNJ 39758979 | H4R | Phase 2a (NCT01497119) | n = 88 | Improvement of histamine-related pruritus. Improvement of Pruritus in moderate AD. | Termination due to agranulocytosis | [9] |
| Adriforant (ZPL-3893787) | H4R | Phase 2 (NCT02424253) | n = 98 | At week 8, Decreased EASI score 50% vs. 27%. Decreased SCORAD score 40% vs 26%. IGA 0/1 18.5% vs. 9.1%. | Completed | [10] |
| Rupatatine | H1R and PAF | Phase 3 (JapicCTI-152787) | n = 66 | Improvement of total pruritus score | Completed | [11] |
| Tezepelumab (plus TCS) | TSLP | Phase 2a (NCT02525094) | n = 113 | At week 12, Pruritus NRS 31.53 vs 21.05. At week 16, IGA response rate 29.4% vs. 12.9%. | Completed | [12] |
| MK8226 | TSLP receptor inhibitor | Phase 1b (NCT01732510) | n = 65 | Results not yet released | Terminate due to business reason | |
| Etokimab (ANB020) | IL-33 | Phase 2a (NCT03533751) | n = 12 | At day 29, EASI 50 83.3%. EASI 75 33%. At day 57, EASI 50 75%. EASI 75 42%. Significant improvement of DLQI and 5D itch scales. | Completed | [13] |
| Etokimab (ANB020) | IL-33 | Phase 2b ATLAS trail | n = 300 | Results not yet released | Recruiting | |
| REGN3500 | IL-33 | Phase 2 (NCT03736967) | n = 206 | Results not yet released | Completed | |
| PF-06817024 | IL-33 | Phase 1 (NCT02743871) | n = 98 | Results not yet released | Completed | |
| Pitrakinra (AER 100, BAY 16-9996) | IL-4 alpha receptor | Phase 2a (NCT00676884) | n = 25 | Results not yet released | Completed | |
| CM310 | IL-4 alpha receptor | Phase 2b (NCT04805411) | n = 120 | Recruiting | Recruiting | |
| CBP-201 | IL-4 alpha receptor | Phase 2 (NCT04444752) | n = 220 | Active, not recruiting | Active, not recruiting | |
| AK210 | IL-4 alpha receptor | Phase 1 (NCT04256174) | n = 70 | Recruiting | Recruiting | |
| Tralokinumab | IL-13 | Phase 3 ECZTRA 1 trial (NCT03131648) | n = 802 | At week 16 IGA 0/1 15.8% vs. 7.1%. EASI 75 25% vs. 12.7. NRS > 4 points improvement 20% vs. 10.9% | Completed | [14] |
| Tralokinumab | IL-13 | Phase 3 ECZTRA 2 trial (NCT03160885) | n = 794 | At week16 IGA 0/1 22.2% vs. 10.9%. EASI 75 33.2% vs. 11.4%. Pruritus NRS > 4 points improvement 25% vs. 9.5%. | Completed | [14] |
| Tralokinumab (plus TCS) | IL-13 | Phase 3 ECZTRA 3 trial(NCT03363854) | n = 380 | At week 16 IGA 0/1 38.9% vs. 26.2%. EASI 75 56% vs. 35.7%. Pruritus NRS > 4 points improvement 45.4% vs. 34.1%. | Completed | [15] |
| Lebrikizumab | IL-13 | Phase 2b (NCT03443024) | n = 280 | At week 16 EASI improvement 72.1% (250 mg q2w) vs. 69.2% (250 mg q4w) vs. 62.3% (125 mg q4w) vs. 41.1% (placebo). Pruritus NRS > 4 points improvement 70% (250mg q2w) vs. 27.5% (placebo). | Completed | [16] |
| Nemolizumab (plus TCS) | IL-31 receptor alpha inhibitor | Phase 2b (NCT03100344) | n = 226 | At week 24 EASI improvement 68.8% vs. 51.2%. PP-NRS reduction 68.6% vs. 34.3% | Completed | [17] |
| Nemolizumab (plus TCS) | IL-31 receptor alpha inhibitor | Phase 3 (JapicCTI number, 173740) | n = 270 | VAS score for pruritus improvement 42.8% vs. 21.4% | Completed | [18] |
| BMS-981164 | IL-31 | Phase 1 (NCT01614756) | n = 93 | Results not yet released | Completed | |
| Tradipitant | Neurokinin receptor | Phase 3 EPIONE trial (NCT03568331) | n = 375 | Improvement of pruritus and sleep in mild lesion AD | Completed | [19] |
| Tradipitant | Neurokinin receptor | Phase 3 EPIONE2 trial (NCT04140695) | n = 200 | Recruiting | Recruiting | |
| Serlopitant | Neurokinin receptor | Phase 2 (NCT02975206) | n = 484 | Failed to meet the primary endpoint WI-NRS score | Completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, L.-S.; Yen, Y.-T.; Lee, C.-H. The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 7227. https://doi.org/10.3390/ijms22137227
Wong L-S, Yen Y-T, Lee C-H. The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis. International Journal of Molecular Sciences. 2021; 22(13):7227. https://doi.org/10.3390/ijms22137227
Chicago/Turabian StyleWong, Lai-San, Yu-Ta Yen, and Chih-Hung Lee. 2021. "The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis" International Journal of Molecular Sciences 22, no. 13: 7227. https://doi.org/10.3390/ijms22137227
APA StyleWong, L.-S., Yen, Y.-T., & Lee, C.-H. (2021). The Implications of Pruritogens in the Pathogenesis of Atopic Dermatitis. International Journal of Molecular Sciences, 22(13), 7227. https://doi.org/10.3390/ijms22137227