Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain
Abstract
:1. Introduction
2. Results
2.1. Molecular Analyses of the Effects of MDPV and α-PVP on Npas4 mRNA Levels
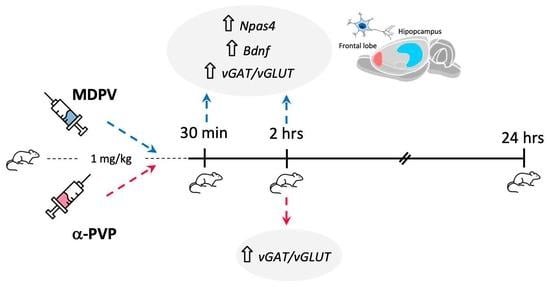
2.1.1. Hippocampus
2.1.2. Frontal Lobe
2.2. Molecular Analysis of the Effects of MDPV and α-PVP on Bdnf mRNA Levels
2.2.1. Hippocampus
2.2.2. Frontal Lobe
2.3. Molecular Analysis of the Effects of MDPV and α-PVP on vGAT1/vGluT1 Ratio
2.3.1. Hippocampus
2.3.2. Frontal Lobe
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug Preparation and Dose Selection
4.3. Analysis of Gene Expression
4.4. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberini, C.M. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009, 89, 121–145. [Google Scholar] [CrossRef]
- Leslie, J.H.; Nedivi, E. Activity-regulated genes as mediators of neural circuit plasticity. Prog. Neurobiol. 2011, 94, 223–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Bloodgood, B.L.; Hauser, J.L.; Lapan, A.D.; Koon, A.C.; Kim, T.K.; Hu, L.S.; Malik, A.N.; Greenberg, M.E. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 2008, 455, 1198–1204. [Google Scholar] [CrossRef]
- Coutellier, L.; Beraki, S.; Ardestani, P.M.; Saw, N.L.; Shamloo, M. Npas4: A neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE 2012, 7, e46604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorthi, K.; Fropf, R.; Belfort, G.M.; Fitzmaurice, H.L.; McKinney, R.M.; Neve, R.L.; Otto, T.; Lin, Y. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 2011, 334, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.J.; Kirkwood, A.; Pizzorusso, T.; Porciatti, V.; Morales, B.; Bear, M.F.; Maffei, L.; Tonegawa, S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999, 98, 739–755. [Google Scholar] [CrossRef] [Green Version]
- Marty, S. Differences in the regulation of neuropeptide Y, somatostatin and parvalbumin levels in hippocampal interneurons by neuronal activity and BDNF. Prog. Brain Res. 2000, 128, 193–202. [Google Scholar] [CrossRef]
- Seil, F.J.; Drake-Baumann, R. Neurotrophins and activity-dependent inhibitory synaptogenesis. Prog. Brain Res. 2000, 128, 219–229. [Google Scholar] [CrossRef]
- Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Mottarlini, F.; Racagni, G.; Brambilla, P.; Fumagalli, F.; Caffino, L. Repeated cocaine exposure during adolescence impairs recognition memory in early adulthood: A role for BDNF signaling in the perirhinal cortex. Dev. Cogn. Neurosci. 2020, 43, 100789. [Google Scholar] [CrossRef]
- Taniguchi, M.; Carreira, M.B.; Cooper, Y.A.; Bobadilla, A.C.; Heinsbroek, J.A.; Koike, N.; Larson, E.B.; Balmuth, E.A.; Hughes, B.W.; Penrod, R.D.; et al. HDAC5 and Its Target Gene, Npas4, Function in the Nucleus Accumbens to Regulate Cocaine-Conditioned Behaviors. Neuron 2017, 96, 130–144.e136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.L.; Xue, B.; Jin, D.Z.; Liu, Z.G.; Fibuch, E.E.; Mao, L.M.; Wang, J.Q. Upregulation of Npas4 protein expression by chronic administration of amphetamine in rat nucleus accumbens in vivo. Neurosci. Lett. 2012, 528, 210–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannotti, G.; Caffino, L.; Calabrese, F.; Racagni, G.; Riva, M.A.; Fumagalli, F. Prolonged abstinence from developmental cocaine exposure dysregulates BDNF and its signaling network in the medial prefrontal cortex of adult rats. Int. J. Neuropsychopharmacol. 2014, 17, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Caputi, F.F.; Caffino, L.; Candeletti, S.; Fumagalli, F.; Romualdi, P. Short-term withdrawal from repeated exposure to cocaine during adolescence modulates dynorphin mRNA levels and BDNF signaling in the rat nucleus accumbens. Drug Alcohol Depend. 2019, 197, 127–133. [Google Scholar] [CrossRef]
- Caffino, L.; Giannotti, G.; Messa, G.; Mottarlini, F.; Fumagalli, F. Repeated cocaine exposure dysregulates BDNF expression and signaling in the mesocorticolimbic pathway of the adolescent rat. World J. Biol. Psychiatry 2019, 20, 531–544. [Google Scholar] [CrossRef]
- Duart-Castells, L.; Lopez-Arnau, R.; Vizcaino, S.; Camarasa, J.; Pubill, D.; Escubedo, E. 7,8-Dihydroxyflavone blocks the development of behavioral sensitization to MDPV, but not to cocaine: Differential role of the BDNF-TrkB pathway. Biochem. Pharmacol. 2019, 163, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinty, J.F.; Whitfield, T.W., Jr.; Berglind, W.J. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010, 1314, 183–193. [Google Scholar] [CrossRef] [Green Version]
- German, C.L.; Fleckenstein, A.E.; Hanson, G.R. Bath salts and synthetic cathinones: An emerging designer drug phenomenon. Life Sci. 2014, 97, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Spiller, H.A.; Ryan, M.L.; Weston, R.G.; Jansen, J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol. 2011, 49, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of Synthetic Cathinones. Handb. Exp. Pharmacol. 2018, 252, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Schneir, A.; Ly, B.T.; Casagrande, K.; Darracq, M.; Offerman, S.R.; Thornton, S.; Smollin, C.; Vohra, R.; Rangun, C.; Tomaszewski, C.; et al. Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin. Toxicol. 2014, 52, 651–658. [Google Scholar] [CrossRef]
- Meyers, K.; Kaynak, O.; Bresani, E.; Curtis, B.; McNamara, A.; Brownfield, K.; Kirby, K.C. The availability and depiction of synthetic cathinones (bath salts) on the Internet: Do online suppliers employ features to maximize purchases? Int. J. Drug Policy 2015, 26, 670–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanciu, C.N.; Penders, T.M.; Gnanasegaram, S.A.; Pirapakaran, E.; Padda, J.S.; Padda, J.S. Withdrawn: The Behavioral Profile of methylenedioxypyrovalerone (MDPV) and alpha-pyrrolidinopentiophenone (PVP)—A Systematic Review. Curr. Drug Abuse Rev. 2017, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zawilska, J.B.; Wojcieszak, J. α-Pyrrolidinophenones: A new wave of designer cathinones. Forensic Toxicol. 2017, 35, 201–216. [Google Scholar] [CrossRef]
- EMCDDA. European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2014: Trends and Developments Luxembourg; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- EMCDDA. European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2016: Trends and Developments Luxembourg; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Glennon, R.A.; Young, R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and alpha-pyrrolidinovalerophenone (alpha-PVP). Brain Res. Bull. 2016, 126, 111–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Health Organization. 3,4-Methylenedioxypyrovalerone (MDPV). Critical Review Report 2014, Agenda item 4.13. In Proceedings of the Expert Committee on Drug Dependence Thirty-sixth Meeting, Geneva, Switzerland, 16–20 June 2014. [Google Scholar]
- WHO. World Health Organization. 1-Phenyl-2-(pyrrolidin-1-yl)pentan-1-one (α-PVP). Critical Review Report 2015, Agenda item 5.3. In Proceedings of the Expert Committee on Drug Dependence Thirty-seventh Meeting, Geneva, Switzerland, 16–20 November 2015. [Google Scholar]
- Smith, D.A.; Blough, B.E.; Banks, M.L. Cocaine-like discriminative stimulus effects of amphetamine, cathinone, methamphetamine, and their 3,4-methylenedioxy analogs in male rhesus monkeys. Psychopharmacology 2017, 234, 117–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, B.A.; Fumagalli, F.; Gainetdinov, R.R.; Jones, S.R.; Ator, R.; Giros, B.; Miller, G.W.; Caron, M.G. Cocaine self-administration in dopamine-transporter knockout mice. Nat. Neurosci. 1998, 1, 132–137. [Google Scholar] [CrossRef]
- Luethi, D.; Kaeser, P.J.; Brandt, S.D.; Krahenbuhl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of methylphenidate-based designer drugs. Neuropharmacology 2018, 134, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Kolanos, R.; Partilla, J.S.; Baumann, M.H.; Hutsell, B.A.; Banks, M.L.; Negus, S.S.; Glennon, R.A. Stereoselective Actions of Methylenedioxypyrovalerone (MDPV) To Inhibit Dopamine and Norepinephrine Transporters and Facilitate Intracranial Self-Stimulation in Rats. ACS Chem. Neurosci. 2015, 6, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, P.C.; Butler, D.; Deschamps, J.R.; Madras, B.K. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: A promising class of monoamine uptake inhibitors. J. Med. Chem. 2006, 49, 1420–1432. [Google Scholar] [CrossRef] [Green Version]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmler, L.D.; Buser, T.A.; Donzelli, M.; Schramm, Y.; Dieu, L.H.; Huwyler, J.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol. 2013, 168, 458–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karila, L.; Lafaye, G.; Scocard, A.; Cottencin, O.; Benyamina, A. MDPV and alpha-PVP use in humans: The twisted sisters. Neuropharmacology 2018, 134, 65–72. [Google Scholar] [CrossRef]
- Fumagalli, F.; Racagni, G.; Colombo, E.; Riva, M.A. BDNF gene expression is reduced in the frontal cortex of dopamine transporter knockout mice. Mol. Psychiatry 2003, 8, 898–899. [Google Scholar] [CrossRef]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef] [Green Version]
- Aarde, S.M.; Creehan, K.M.; Vandewater, S.A.; Dickerson, T.J.; Taffe, M.A. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: Self-administration and locomotor stimulation in male rats. Psychopharmacology 2015, 232, 3045–3055. [Google Scholar] [CrossRef] [Green Version]
- Gatch, M.B.; Dolan, S.B.; Forster, M.J. Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J. Pharmacol. Exp. Ther. 2015, 354, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Gatch, M.B.; Taylor, C.M.; Forster, M.J. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav. Pharmacol. 2013, 24, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marusich, J.A.; Grant, K.R.; Blough, B.E.; Wiley, J.L. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology 2012, 33, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- De-Giorgio, F.; Bilel, S.; Ossato, A.; Tirri, M.; Arfe, R.; Foti, F.; Serpelloni, G.; Frisoni, P.; Neri, M.; Marti, M. Acute and repeated administration of MDPV increases aggressive behavior in mice: Forensic implications. Int. J. Legal. Med. 2019, 133, 1797–1808. [Google Scholar] [CrossRef]
- De-Giorgio, F.; Bilel, S.; Ossato, A.; Tirri, M.; Arfe, R.; Foti, F.; Serpelloni, G.; Frisoni, P.; Neri, M.; Marti, M. Reply to “MDPV-induced aggression in humans not established”. Int. J. Legal Med. 2020, 134, 263–265. [Google Scholar] [CrossRef]
- Foti, F.; Bilel, S.; Tirri, M.; Arfe, R.; Boccuto, F.; Bernardi, T.; Serpelloni, G.; de-Giorgio, F.; Marti, M. Low-normal doses of methiopropamine induce aggressive behaviour in mice. Psychopharmacology 2021, 238, 1847–1856. [Google Scholar] [CrossRef]
- Bonano, J.S.; Glennon, R.A.; de Felice, L.J.; Banks, M.L.; Negus, S.S. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology 2014, 231, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantegrossi, W.E.; Gannon, B.M.; Zimmerman, S.M.; Rice, K.C. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 2013, 38, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Naylor, J.E.; Freeman, K.B.; Blough, B.E.; Woolverton, W.L.; Huskinson, S.L. Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend. 2015, 149, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannotti, G.; Canazza, I.; Caffino, L.; Bilel, S.; Ossato, A.; Fumagalli, F.; Marti, M. The Cathinones MDPV and alpha-PVP Elicit Different Behavioral and Molecular Effects Following Acute Exposure. Neurotox. Res. 2017, 32, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Mottarlini, F.; Mingardi, J.; Zita, G.; Barbon, A.; Fumagalli, F. Anhedonic-like behavior and BDNF dysregulation following a single injection of cocaine during adolescence. Neuropharmacology 2020, 175, 108161. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Messa, G.; Fumagalli, F. A single cocaine administration alters dendritic spine morphology and impairs glutamate receptor synaptic retention in the medial prefrontal cortex of adolescent rats. Neuropharmacology 2018, 140, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fremeau, R.T., Jr.; Voglmaier, S.; Seal, R.P.; Edwards, R.H. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004, 27, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, F.; di Pasquale, L.; Caffino, L.; Racagni, G.; Riva, M.A. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur. J. Neurosci. 2007, 26, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Hinton, E.A.; Wheeler, M.G.; Gourley, S.L. Early-life cocaine interferes with BDNF-mediated behavioral plasticity. Learn Mem. 2014, 21, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fumagalli, F.; Moro, F.; Caffino, L.; Orru, A.; Cassina, C.; Giannotti, G.; di Clemente, A.; Racagni, G.; Riva, M.A.; Cervo, L. Region-specific effects on BDNF expression after contingent or non-contingent cocaine i.v. self-administration in rats. Int. J. Neuropsychopharmacol. 2013, 16, 913–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, J.M.; Taylor, J.R.; de Vries, T.J.; Peters, J. Brain-derived neurotrophic factor and addiction: Pathological versus therapeutic effects on drug seeking. Brain Res. 2015, 1628, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Pitts, E.G.; Taylor, J.R.; Gourley, S.L. Prefrontal cortical BDNF: A regulatory key in cocaine- and food-reinforced behaviors. Neurobiol. Dis. 2016, 91, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Giannotti, G.; Barry, S.M.; Siemsen, B.M.; Peters, J.; McGinty, J.F. Divergent Prelimbic Cortical Pathways Interact with BDNF to Regulate Cocaine-seeking. J. Neurosci. 2018, 38, 8956–8966. [Google Scholar] [CrossRef]
- Fumagalli, F.; Caffino, L.; Racagni, G.; Riva, M.A. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur. Neuropsychopharmacol. 2009, 19, 402–408. [Google Scholar] [CrossRef]
- Fumagalli, F.; Franchi, C.; Caffino, L.; Racagni, G.; Riva, M.A.; Cervo, L. Single session of cocaine intravenous self-administration shapes goal-oriented behaviours and up-regulates Arc mRNA levels in rat medial prefrontal cortex. Int. J. Neuropsychopharmacol. 2009, 12, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.J.; Zou, M.; Lu, L.; Lau, D.; Ditzel, D.A.; Delucinge-Vivier, C.; Aso, Y.; Descombes, P.; Bading, H. Nuclear calcium signaling controls expression of a large gene pool: Identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009, 5, e1000604. [Google Scholar] [CrossRef]
- Rudin, D.; Liechti, M.E.; Luethi, D. Molecular and clinical aspects of potential neurotoxicity induced by new psychoactive stimulants and psychedelics. Exp. Neurol. 2021, 343, 113778. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A-PVP, P. Available online: https://psychonautwiki.org/wiki/A-PVP (accessed on 21 May 2021).
- MDPV, P. Available online: https://psychonautwiki.org/wiki/MDPV (accessed on 21 May 2021).
- Erowid. Available online: https://erowid.org/chemicals/mdpv/mdpv_dose.shtml (accessed on 21 May 2021).
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates: Compact, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2004. [Google Scholar]
- Spijker, S. Dissection of Rodent Brain Regions. In Neuroproteomics; Li, K.W., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 13–26. [Google Scholar] [CrossRef]
- Caffino, L.; Giannotti, G.; Malpighi, C.; Racagni, G.; Fumagalli, F. Short-term withdrawal from developmental exposure to cocaine activates the glucocorticoid receptor and alters spine dynamics. Eur. Neuropsychopharmacol. 2015, 25, 1832–1841. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caffino, L.; Mottarlini, F.; Bilel, S.; Targa, G.; Tirri, M.; Maggi, C.; Marti, M.; Fumagalli, F. Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain. Int. J. Mol. Sci. 2021, 22, 7397. https://doi.org/10.3390/ijms22147397
Caffino L, Mottarlini F, Bilel S, Targa G, Tirri M, Maggi C, Marti M, Fumagalli F. Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain. International Journal of Molecular Sciences. 2021; 22(14):7397. https://doi.org/10.3390/ijms22147397
Chicago/Turabian StyleCaffino, Lucia, Francesca Mottarlini, Sabrine Bilel, Giorgia Targa, Micaela Tirri, Coralie Maggi, Matteo Marti, and Fabio Fumagalli. 2021. "Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain" International Journal of Molecular Sciences 22, no. 14: 7397. https://doi.org/10.3390/ijms22147397
APA StyleCaffino, L., Mottarlini, F., Bilel, S., Targa, G., Tirri, M., Maggi, C., Marti, M., & Fumagalli, F. (2021). Single Exposure to the Cathinones MDPV and α-PVP Alters Molecular Markers of Neuroplasticity in the Adult Mouse Brain. International Journal of Molecular Sciences, 22(14), 7397. https://doi.org/10.3390/ijms22147397