Anti-Inflammatory Effect of Very High Dose Local Vessel Wall Statin Administration: Poly(L,L-Lactide) Biodegradable Microspheres with Simvastatin for Drug Delivery System (DDS)
Abstract
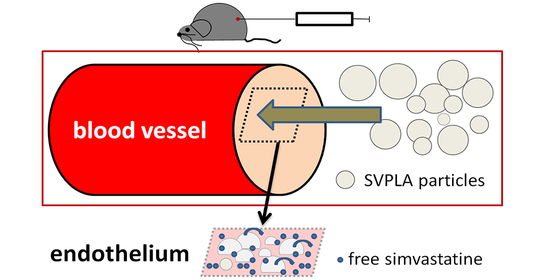
:1. Introduction
2. Results
2.1. SVPLA Microspheres
2.2. Evaluation of the Release Rate of SV from SVPLA Particles Investigated In Vitro
2.3. Quantitative Assessment of SV Deposition When Administered to a Vessel
2.4. Evaluation of Deposition of PLA-DH Microspheres on the Vessel Wall
2.5. Histopathology of the Liver, Heart, Lung and Kidney of Rats after SVPLA Microsphere Administration
2.6. Effect of Microsphere SVPLA Inflammation of the Vessel Wall in an Animal Model (Rat) 24 h, 7 Days and 4 Weeks after Administration
3. Materials and Methods
3.1. Drugs and Other Substances Used in the Studies
3.2. Poly(L,L-Lactide) Particles Loaded with Simvastatin (SVPLA) and/or Poly(L,L-Lactide) Particles with Fluorescent Dansylhydrazine Used in the Studies
3.3. Determination of the Release of Simvastatin (SV) from SVPLA Particles Investigated In Vitro
3.4. Studies of SVPLA Microsphere Delivery to Biological Material (In Vitro, In Vivo)
The Effect of Simvastatin Administered in the Form of Microspheres (SVPLA) into Inflamed Vascular Endothelium (the Main Study)
3.5. Characteristics of Animal Groups
3.6. Deposition Test of PLA Particles Labeled with Fluorescent Dansylhydrazine (PLA-DH) in the Wall of the Vessel
3.7. Measurements of Biochemical Parameters in Serum
3.8. Histopathological and Immunohistochemical Studies
3.9. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Hansson, G.K. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. The molecular mechanisms of the thrombotic complications of atherosclerosis. J. Intern. Med. 2008, 263, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Drexler, H. The clinical significance of endothelial dysfunction. Curr. Opin. Cardiol. 2005, 20, 547–551. [Google Scholar] [CrossRef]
- Clark, R.M.; Andrew, L.; Doehner, W.; Davos, C.; Aidan, B.; Rakesh, S.; Andrew, J.S.C.; Stefan, D.A. The relationship between cholesterol and survival in patients with chronic heart failure. Am. J. Coll. Cardiol. 2003, 42, 1941–1943. [Google Scholar]
- Schachter, M. Fundamental & Clinical Pharmacology; Blackwell Publishing: Hoboken, NJ, USA, 2004; Volume 19, pp. 117–125. [Google Scholar]
- Persson, E.M.; Gustafsson, A.-S.; Carlsson, A.S.; Nilsson, R.G.; Knutson, L.; Forsell, P.; Hanisch, G.; Lennernäs, H.; Abrahamsson, B. The Effects of Food on the Dissolution of Poorly Soluble Drugs in Human and in Model Small Intestinal Fluids. Pharm. Res. 2005, 22, 2141–2151. [Google Scholar] [CrossRef]
- Sudoh, M.; Kishimoto, Y.; Marumoto, A.; Inoue, M.; Sano, A.; Miura, N.; Horie, Y.; Hasegawa, J.; Ryoke, K. A new animal model of continuous catheterization for investigating mechanisms of arteritis associated with chemotherapy. Life Sci. 2004, 74, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Vicaut, E.; Stucker, O. An Intact Muscle Preparation for Studying the Microcirculation by in vivo Microscopy. Micro-Vasc. Res. 1990, 39, 120–122. [Google Scholar] [CrossRef]
- Laemmel, E.; Bonnardel-Phu, E.; Hou, X.; Seror, J.; Vicaut, E. Interaction between nitric oxide and prostanoids in arterioles of rat cremaster muscle in vivo. Am. J. Physiol. Circ. Physiol. 2003, 285, H1254–H1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacinski, P.; Gadzinowski, M.; Marciniak, A.; Dąbrowski, W.; Szumiło, J.; Wysokiński, A.; Czuczwar, S.; Slomkowski, S. A direct local drug delivery. A nanotechnology-based local drug delivery using biodegradable nanoparticles. Am. J. Cardiol. 2008, 102, 182. [Google Scholar]
- Gill, G.W. Pathology-Guide. Special Stains and H and E; DAKO: Glostrup, Denmark, 2010; Volume 20, pp. 177–185. [Google Scholar]
- Decuzzi, P.; Pasqualini, R.; Arap, W.; Ferrari, M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm Res. 2008, 26, 236–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, B.S.; Dziubla, T.; Shuvaev, V.V.; Muro, S.; Muzykantov, V.R. Advanced drug delivery systems that target the vas-cular endothelium. Mol. Interv. 2006, 6, 98–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decuzzi, P.; Lee, S.; Bhushan, B.; Ferrari, M. A Theoretical Model for the Margination of Particles within Blood Vessels. Ann. Biomed. Eng. 2005, 33, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 2006, 27, 5307–5314. [Google Scholar] [CrossRef]
- Jacobson, T.A. Myopathy with statin–fibrate combination therapy: Clinical considerations. Nat. Rev. Endocrinol. 2009, 5, 507–518. [Google Scholar] [CrossRef]
- Stroes, E.; Thompson, P.D.; Corsini, A. Statin-associated muscle symptoms: Impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Etiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Igel, M.; Sudhop, T.; Von Bergmann, K. Metabolism and drug interactions of 3-hydroxy-3-methylglutaryl coenzyme A-reductase inhibitors (statins). Eur. J. Clin. Pharmacol. 2001, 57, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2004, 19, 311–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- lllum, L.; Davis, S.S. The targeting of drugs parenterally by use of microspheres. J. Parenter Sci. Tech. 1992, 36, 242–248. [Google Scholar]
- Strobel, H.A.; Qendro, E.I.; Alsberg, E.; Rolle, M.W. Targeted Delivery of Bioactive Molecules for Vascular Intervention and Tissue Engineering. Front. Pharmacol. 2018, 9, 1329. [Google Scholar] [CrossRef] [PubMed]










| Parameter | The Microspheres Used in the Study |
|---|---|
| The number of microspheres measured, N | 301 |
| The largest microsphere measured, μm | 17.0 |
| The smallest microsphere measured, μm | 0.22 |
| The average diameter of the microspheres, μm (± SD) | 6.40 ± 3.28 |
| Sample Number | SVPLA A (μg/mg) 1 | SVPLA B (μg/mg) | SVPLA C (μg/mg) | Control 2 SV per os |
|---|---|---|---|---|
| 1 | 18.34 | 27.21 | 10.47 | 0 |
| 2 | 8.58 | 16.74 | 8.58 | 0 |
| 3 | 33.15 | 27.21 | 11.15 | 0 |
| 4 | 23.60 | 30.72 | 16.22 | 0 |
| 5 | 23.11 | 28.25 | 15.01 | 0 |
| 6 | 24.96 | 39.04 | 24.41 | 0 |
| Average ± SD | 21.95 ± 8.12 | 28.19 ± 7.17 | 14.30 ± 5.72 | 0 |
| Group # | T | Min | Max | Mean ± SD |
|---|---|---|---|---|
| SVPLA 25% | 24 h | 1.12 | 1.76 | 1.26 ± 0.21 * |
| 7 days | 1.08 | 1.54 | 1.24 ± 0.16 * | |
| 28 days | 0.99 | 1.32 | 1.21 ± 0.21 * | |
| SVPLA 50% | 24 h | 1.01 | 1.97 | 1.29 ± 0.28 * |
| 7 days | 0.99 | 1.87 | 1.31 ± 0.31 * | |
| 28 days | 0.96 | 1.45 | 1.18 ± 0.21 * | |
| SV with food | 24 h | 0.91 | 1.43 | 1.09 ± 0.18 |
| 7 days | 0.80 | 1.21 | 0.94 ± 0.14 ** | |
| 28 days | 0.76 | 0.98 | 0.86 ± 0.14 ** | |
| Control | 24 h | 1.09 | 1.68 | 1.22 ± 0.12 |
| 7 days | 1.08 | 1.78 | 1.30 ± 0.21 | |
| 28 days | 1.02 | 1.57 | 1.23 ± 0.21 |
| Main Study Flow Chart | ||||
|---|---|---|---|---|
| Day-3, 2, 1 | Para Vessel PTX/5-FU Injections n = 72 | |||
| Day 0 | SVPLA 50% n = 18 | SVPLA 25% n = 18 | SV * (0.58 mg/kg) n = 18 | Control n = 18 |
| 24 h *** | MP ** (vessel wall) n = 6 | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 |
| 7 Days | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 |
| 4 Weeks | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 | MP (vessel wall) n = 6 |
| SVPLA Dispersion Concentration (mg/mL) | Recipe | SV Loading Dose/kg | SV Solution Volume Administered (mL) per Animal | |
|---|---|---|---|---|
| Solution A | 0.3500 | 1 mL A | 0.58 mg | 1 |
| Solution B | 0.1750 | 1 mL A + 1 mL PBS | 0.58 mg | 2 |
| Solution C | 0.0875 | 1 mL A + 3 mL PBS | 0.58 mg | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacinski, P.; Gadzinowski, M.; Dabrowski, W.; Szumilo, J.; Wacinski, J.; Oru, N.; Vicaut, E.; Czuczwar, S.; Kocki, J.; Basinska, T.; et al. Anti-Inflammatory Effect of Very High Dose Local Vessel Wall Statin Administration: Poly(L,L-Lactide) Biodegradable Microspheres with Simvastatin for Drug Delivery System (DDS). Int. J. Mol. Sci. 2021, 22, 7486. https://doi.org/10.3390/ijms22147486
Wacinski P, Gadzinowski M, Dabrowski W, Szumilo J, Wacinski J, Oru N, Vicaut E, Czuczwar S, Kocki J, Basinska T, et al. Anti-Inflammatory Effect of Very High Dose Local Vessel Wall Statin Administration: Poly(L,L-Lactide) Biodegradable Microspheres with Simvastatin for Drug Delivery System (DDS). International Journal of Molecular Sciences. 2021; 22(14):7486. https://doi.org/10.3390/ijms22147486
Chicago/Turabian StyleWacinski, Piotr, Mariusz Gadzinowski, Wojciech Dabrowski, Justyna Szumilo, Jakub Wacinski, Nathalie Oru, Eric Vicaut, Stanislaw Czuczwar, Janusz Kocki, Teresa Basinska, and et al. 2021. "Anti-Inflammatory Effect of Very High Dose Local Vessel Wall Statin Administration: Poly(L,L-Lactide) Biodegradable Microspheres with Simvastatin for Drug Delivery System (DDS)" International Journal of Molecular Sciences 22, no. 14: 7486. https://doi.org/10.3390/ijms22147486
APA StyleWacinski, P., Gadzinowski, M., Dabrowski, W., Szumilo, J., Wacinski, J., Oru, N., Vicaut, E., Czuczwar, S., Kocki, J., Basinska, T., & Slomkowski, S. (2021). Anti-Inflammatory Effect of Very High Dose Local Vessel Wall Statin Administration: Poly(L,L-Lactide) Biodegradable Microspheres with Simvastatin for Drug Delivery System (DDS). International Journal of Molecular Sciences, 22(14), 7486. https://doi.org/10.3390/ijms22147486