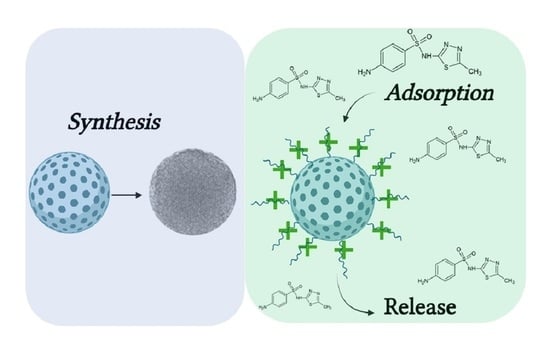
Adsorption and Release of Sulfamethizole from Mesoporous Silica Nanoparticles Functionalised with Triethylenetetramine
Abstract
:1. Introduction
2. Results
2.1. MSN Structural Characterisation
2.2. SMZ Adsorption on MSN and MSN-TETA
2.3. Adsorption Kinetics of Sulphamethizole on MCM-41 and MCM-41-TETA
2.4. Adsorption Isotherms of Sulphamethizole on MCM-41 and MCM-41-TETA
2.5. In Vitro Release of SMZ
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Functionalisation of MSNs
3.3. Physico-Chemical Characterisations
3.4. Adsorption Kinetic Models
3.5. Adsorption Isotherms
3.6. Kinetics of SMZ Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Piludu, M.; Medda, L.; Monduzzi, M.; Salis, A. Gold Nanoparticles: A Powerful Tool to Visualize Proteins on Ordered Mesoporous Silica and for the Realization of Theranostic Nanobioconjugates. Int. J. Mol. Sci. 2018, 19, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Zhao, W.; Tan, W.; Lai, Z.; Fang, D.; Jiang, L.; Zuo, C.; Yang, N.; Lai, Y. An Efficient Cell-Targeting Drug Delivery System Based on Aptamer-Modified Mesoporous Silica Nanoparticles. Nanoscale Res. Lett. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.G.; Surendran, S.P.; Jeong, Y.Y. Tumor Microenvironment-Stimuli Responsive Nanoparticles for Anticancer Therapy. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, X.; Soeriyadi, A.; Sagnella, S.M.; Lu, X.; Scott, J.; Lowe, S.B.; Kavallaris, M.; Gooding, J.J. Stimuli-responsive functionalized mesoporous silica nanoparticles for drug release in response to various biological stimuli. Biomater. Sci. 2014, 2, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regi, M.; Rámila, A.; DEL Real, R.P.; Perez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Latterini, L.; Marmottini, F.; Ricci, M.; Rossi, C. MCM-41 for furosemide dissolution improvement. Microporous Mesoporous Mater. 2012, 147, 343–349. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Saviot, L.; Bouyer, F.; Baras, F.; Bouyer, F. Optimization of MCM-41 type silica nanoparticles for biological applications: Control of size and absence of aggregation and cell cytotoxicity. J. Non Cryst. Solids 2015, 408, 87–97. [Google Scholar] [CrossRef]
- Popova, T.; Voycheva, C.; Tzankov, B. Study on the influence of technological factors on drug loading of poorly water-soluble drug on MCM-41 mesoporous carrier. Pharmacia 2020, 67, 351–356. [Google Scholar] [CrossRef]
- Roik, N.; Belyakova, L.A.; Dziazko, M.O. Adsorption of antitumor antibiotic doxorubicin on MCM-41-type silica surface. Adsorpt. Sci. Technol. 2017, 35, 86–101. [Google Scholar] [CrossRef]
- He, Q.; Shi, J. MSN Anti-Cancer Nanomedicines: Chemotherapy Enhancement, Overcoming of Drug Resistance, and Metastasis Inhibition. Adv. Mater. 2014, 26, 391–411. [Google Scholar] [CrossRef] [PubMed]
- LaBauve, A.E.; Rinker, T.E.; Noureddine, A.; Serda, R.E.; Howe, J.Y.; Sherman, M.B.; Rasley, A.; Brinker, C.J.; Sasaki, D.Y.; Negrete, O.A. Lipid-Coated Mesoporous Silica Nanoparticles for the Delivery of the ML336 Antiviral to Inhibit Encephalitic Alphavirus Infection. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohseni, M.; Gilani, K.; Mortazavi, S.A. Preparation and Characterization of Rifampin Loaded Mesoporous Silica Nanoparticles as a Potential System for Pulmonary Drug Delivery. Iran. J. Pharm. Res. 2015, 14, 27–34. [Google Scholar] [CrossRef]
- Ganesh, M.; Gil Lee, S. Synthesis, Characterization and Drug Release Capability of New Cost Effective Mesoporous Silica Nano Particle for Ibuprofen Drug Delivery. Int. J. Control. Autom. 2013, 6, 207–216. [Google Scholar] [CrossRef]
- Alexa, I.F.; Ignat, M.; Popovici, R.F.; Timpu, D.; Popovici, E. In vitro controlled release of antihypertensive drugs intercalated into unmodified SBA-15 and MgO modified SBA-15 matrices. Int. J. Pharm. 2012, 436, 111–119. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Izquierdo-Barba, I.; Colilla, M.; Vallet-Regí, M. Concanavalin A-targeted mesoporous silica nanoparticles for infection treatment. Acta Biomater. 2019, 96, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Fanti, M.; Medda, L.; Nairi, V.; Cugia, F.; Piludu, M.; Sogos, V.; Monduzzi, M. Mesoporous Silica Nanoparticles Functionalized with Hyaluronic Acid and Chitosan Biopolymers. Effect of Functionalization on Cell Internalization. ACS Biomater. Sci. Eng. 2016, 2, 741–751. [Google Scholar] [CrossRef]
- Nairi, V.; Magnolia, S.; Piludu, M.; Nieddu, M.; Caria, C.A.; Sogos, V.; Vallet-Regì, M.; Monduzzi, M.; Salis, A. Mesoporous silica nanoparticles functionalized with hyaluronic acid. Effect of the biopolymer chain length on cell internalization. Colloids Surf. B Biointerfaces 2018, 168, 50–59. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regí, M.; Monduzzi, M.; Salis, A. Interactions between bovine serum albumin and mesoporous silica nanoparticles functionalized with biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmacia 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Li, Z.; Zink, J.I.; Tamanoi, F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: Enhanced efficacy by folate modification. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.C.; Nguyen, C.T.H.; Strounina, E.; Davis-Poynter, N.; Ross, B.P. Structure–Activity Relationships of GAG Mimetic-Functionalized Mesoporous Silica Nanoparticles and Evaluation of Acyclovir-Loaded Antiviral Nanoparticles with Dual Mechanisms of Action. ACS Omega 2018, 3, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Gounani, Z.; Asadollahi, M.A.; Meyer, R.L.; Arpanaei, A. Loading of polymyxin B onto anionic mesoporous silica nanoparticles retains antibacterial activity and enhances biocompatibility. Int. J. Pharm. 2018, 537, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ledingham, K. Using a cultural contexts of health approach to address the challenge of antibiotic resistance. Eur. J. Public Health 2019, 29. [Google Scholar] [CrossRef]
- Alanis, A.J. Resistance to Antibiotics: Are We in the Post-Antibiotic Era? Arch. Med Res. 2005, 36, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Labruère, R.; Sona, A.J.; Turos, E. Anti–Methicillin-Resistant Staphylococcus aureus Nanoantibiotics. Front. Pharmacol. 2019, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.C. Mechanisms of bacterial resistance to antimicrobial agents. Am. J. Health Pharm. 1997, 54, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Parasca, O.M.; Gheaţǎ, F.; Pânzariu, A.; Geangalǎu, I.; Profire, L. Importance of Sulfonamide Moiety in Current and Future Therapy. Rev. Med. Chir. Soc. Medici Nat. Iaş̧i 2013, 117, 558–564. [Google Scholar]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.M. The Biosynthesis of Folic Acid. J. Biol. Chem. 1962, 237, 536–540. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.; Sharma, S.K.; Hashmi, J.T.; Kurup, D.B.; Hamblin, M.R. Topical antimicrobials for burn wound infections. Recent Pat. Anti Infect. Drug Discov. 2010, 5, 124–151. [Google Scholar] [CrossRef] [Green Version]
- Peddie, B.A.; Little, P.J.; Peddle, B.A. Sulphamethizole in urinary tract infections. J. Antimicrob. Chemother. 1979, 5, 195–200. [Google Scholar] [CrossRef]
- Ghedini, E.; Pizzolitto, C.; Albore, G.; Menegazzo, F.; Signoretto, M.; Operti, L.; Cerrato, G. Sulfadiazine-based drug delivery systems prepared by an effective sol?gel process. J. Sol Gel Sci. Technol. 2017, 83, 618–626. [Google Scholar] [CrossRef]
- Águila-Rosas, J.; Quirino-Barreda, C.T.; Leyva-Gómez, G.; González-Zamora, E.; Ibarra, I.A.; Lima, E. Sulfadiazine hosted in MIL-53(Al) as a biocide topical delivery system. RSC Adv. 2020, 10, 25645–25651. [Google Scholar] [CrossRef]
- Jangra, S.; Devi, S.; Tomer, V.K.; Chhokar, V.; Duhan, S. Improved antimicrobial property and controlled drug release kinetics of silver sulfadiazine loaded ordered mesoporous silica. J. Asian Ceram. Soc. 2016, 4, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Giret, S.; Man, M.W.C.; Carcel, C. Mesoporous-Silica-Functionalized Nanoparticles for Drug Delivery. Chem. A Eur. J. 2015, 21, 13850–13865. [Google Scholar] [CrossRef]
- Musso, G.E.; Bottinelli, E.; Celi, L.R.; Magnacca, G.; Berlier, G. Influence of surface functionalization on the hydrophilic character of mesoporous silica nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 13882–13894. [Google Scholar] [CrossRef]
- Montalvo-Quirós, S.; Vallet-Regí, M.; Palacios, A.; Anguita, J.; Prados-Rosales, R.C.; González, B.; Luque-Garcia, J.L. Mesoporous Silica Nanoparticles as a Potential Platform for Vaccine Development against Tuberculosis. Pharmceutics 2020, 12, 1218. [Google Scholar] [CrossRef]
- Fulaz, S.; Devlin, H.; Vitale, S.; Quinn, L.; O’Gara, J.P.; Casey, E. Tailoring Nanoparticle-Biofilm Interactions to Increase the Efficacy of Antimicrobial Agents Against Staphylococcus aureus. Int. J. Nanomed. 2020, ume 15, 4779–4791. [Google Scholar] [CrossRef]
- Zaharudin, N.S.; Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Jumbri, K. Functionalized mesoporous silica nanoparticles templated by pyridinium ionic liquid for hydrophilic and hydrophobic drug release application. J. Saudi Chem. Soc. 2020, 24, 289–302. [Google Scholar] [CrossRef]
- Niu, J.; Tang, G.; Tang, J.; Yang, J.; Zhou, Z.; Gao, Y.; Chen, X.; Tian, Y.; Li, Y.; Li, J.; et al. Functionalized Silver Nanocapsules with Improved Antibacterial Activity Using Silica Shells Modified with Quaternary Ammonium Polyethyleneimine as a Bacterial Cell-Targeting Agent. J. Agric. Food Chem. 2021, 69, 6485–6494. [Google Scholar] [CrossRef]
- Mas, N.; Galiana, I.; Mondragón, L.; Aznar, E.; Climent, E.; Cabedo, N.; Sancenón, F.; Murguía, J.R.; Martinez, L.M.; Marcos, M.D.; et al. Enhanced Efficacy and Broadening of Antibacterial Action of Drugs via the Use of Capped Mesoporous Nanoparticles. Chem. A Eur. J. 2013, 19, 11167–11171. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crisponi, G.; Crespo-Alonso, M.; Lachowicz, J.I.; Szewczuk, Z.; Cooper, G.J.S. Complex formation equilibria of CuIIand ZnIIwith triethylenetetramine and its mono- and di-acetyl metabolites. Dalton Trans. 2013, 42, 6161–6170. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Delpiano, G.R.; Zanda, D.; Piludu, M.; Sanjust, E.; Monduzzi, M.; Salis, A. Adsorption of Cu2+ and Zn2+ on SBA-15 mesoporous silica functionalized with triethylenetetramine chelating agent. J. Environ. Chem. Eng. 2019, 7, 103205. [Google Scholar] [CrossRef]
- Kim, S.T.; Chompoosor, A.; Yeh, Y.-C.; Agasti, S.; Solfiell, D.J.; Rotello, V.M. Dendronized Gold Nanoparticles for siRNA Delivery. Small 2012, 8, 3253–3256. [Google Scholar] [CrossRef] [Green Version]
- Gottenbos, B.; Grijpma, D.W.; Van Der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.F.; Garcia-Uriostegui, L.; Rodríguez, O.; Izquierdo-Barba, I.; Salinas, A.J.; Toriz, G.; Vallet-Regí, M.; Delgado, E. Lysine-Grafted MCM-41 Silica as an Antibacterial Biomaterial. Bioengineering 2017, 4, 80. [Google Scholar] [CrossRef] [Green Version]
- Lowe, B.M.; Skylaris, C.-K.; Green, N.G. Acid-base dissociation mechanisms and energetics at the silica–water interface: An activationless process. J. Colloid Interface Sci. 2015, 451, 231–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apella, M.C.; Baran, E.J. The Infrared Spectra of Some Sulfate-Apatites. Spectrosc. Lett. 1979, 12, 1–6. [Google Scholar] [CrossRef]
- Dindar, M.H.; Yaftian, M.R.; Rostamnia, S. Potential of functionalized SBA-15 mesoporous materials for decontamination of water solutions from Cr(VI), As(V) and Hg(II) ions. J. Environ. Chem. Eng. 2015, 3, 986–995. [Google Scholar] [CrossRef]
- Fukahori, S.; Fujiwara, T.; Ito, R.; Funamizu, N. pH-Dependent adsorption of sulfa drugs on high silica zeolite: Modeling and kinetic study. Desalination 2011, 275, 237–242. [Google Scholar] [CrossRef]
- Medda, L.; Casula, M.F.; Monduzzi, M.; Salis, A. Adsorption of Lysozyme on Hyaluronic Acid Functionalized SBA-15 Mesoporous Silica: A Possible Bioadhesive Depot System. Langmuir 2014, 30, 12996–13004. [Google Scholar] [CrossRef]
- Ho, Y.-S. Selection of optimum sorption isotherm. Carbon 2004, 42, 2115–2116. [Google Scholar] [CrossRef]
- Jia, X.; Li, S.; Wang, Y.; Ang, T.W.; Hou, X. Adsorption Behavior and Mechanism of Sulfonamide Antibiotics in Aqueous Solution on a Novel MIL-101(Cr)@GO Composite. J. Chem. Eng. Data 2019, 64, 1265–1274. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.; McNeill, K. Photochemical Fate of Sulfa Drugs in the Aquatic Environment: Sulfa Drugs Containing Five-Membered Heterocyclic Groups. Environ. Sci. Technol. 2004, 38, 3933–3940. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lo, L.-W.; Mou, C.-Y.; Yang, C.-S. Synthesis and Characterization of Positive-Charge Functionalized Mesoporous Silica Nanoparticles for Oral Drug Delivery of an Anti-Inflammatory Drug. Adv. Funct. Mater. 2008, 18, 3283–3292. [Google Scholar] [CrossRef]
- Climent, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Maquieira, A.; Amorós, P. Controlled Delivery Using Oligonucleotide-Capped Mesoporous Silica Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7281–7283. [Google Scholar] [CrossRef]
- FDA. Trimethoprim with sulfamethoxazole (Bactrim) in gastroenterology [TRIMETHOPRIM SULFAMETHOXAZOL (BACTRIM) IN DER GASTROENTEROLOGIE]. Praxis 1974, 63, 680–684. [Google Scholar]
- Yoshikawa, T.T.; Guze, L.B. Concentrations of Trimethoprim-Sulfamethoxazole in Blood After a Single, Large Oral Dose. Antimicrob. Agents Chemother. 1976, 10, 462–463. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.E.; Rieder, J. Pharmacokinetics of Sulfamethoxazole plus Trimethoprim in Man and Their Distribution in the Rat. Chemotherapy 1970, 15, 337–355. [Google Scholar] [CrossRef]
- Dao, B.D.; Barreto, J.N.; Wolf, R.C.; Dierkhising, R.A.; Plevak, M.F.; Tosh, P.K. Serum peak sulfamethoxazole concentrations demonstrate difficulty in achieving a target range: A retrospective cohort study. Curr. Ther. Res. 2014, 76, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Lachowicz, J.I.; Emwas, A.; Delpiano, G.R.; Salis, A.; Piludu, M.; Jaremko, L.; Jaremko, M. Improving Metal Adsorption on Triethylenetetramine (TETA) Functionalized SBA-15 Mesoporous Silica Using Potentiometry, EPR and ssNMR. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Da’Na, E.; De Silva, N.; Sayari, A. Adsorption of copper on amine-functionalized SBA-15 prepared by co-condensation: Kinetics properties. Chem. Eng. J. 2011, 166, 454–459. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, X.; Gu, J.; Zhao, L.; Jiang, L.; Qiu, Y. Effective adsorption and concentration of carnosine by nickel species within mesoporous silica. LWT 2016, 74, 211–218. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Hajati, S.; Mehrabi, F.; Goudarzi, A. Preparation of nanomaterials for the ultrasound-enhanced removal of Pb2+ ions and malachite green dye: Chemometric optimization and modeling. Ultrason. Sonochemistry 2017, 34, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Steri, D.; Monduzzi, M.; Salis, A. Ionic strength affects lysozyme adsorption and release from SBA-15 mesoporous silica. Microporous Mesoporous Mater. 2013, 170, 164–172. [Google Scholar] [CrossRef]
- Nieto, A.; Colilla, M.; Balas, F.; Vallet-Regí, M. Surface Electrochemistry of Mesoporous Silicas as a Key Factor in the Design of Tailored Delivery Devices. Langmuir 2010, 26, 5038–5049. [Google Scholar] [CrossRef]








| Sample | a SBET (m2g−1) | b Vp (cm3g−1) | c dp (Å) | da (Å) | e ζ (mV) | f L (µmol g−1) |
|---|---|---|---|---|---|---|
| MSN | 942 | 0.78 | 25 | 48 | −23 | - |
| MSN-Cl | 744 | 0.77 | 25 | 47 | −8 | 770 |
| MSN-TETA | 671 | 0.62 | 24 | 46 | +27 | 469 |
| Pseudo-First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|
| Sample | qeqexp (mg g−1) | k’ (min−1) | qeqcalc (mg g−1) | R2 | k” (g mg−1 min−1) | qeqcalc (mg g−1) | R2 |
| MSN-SMZ | 232.9 | 4.8 × 10−4 | 81.3 | 0.17 | 6.4 × 10−4 | 216.2 | 0.99 |
| MSN-TETA-SMZ | 389.1 | 1.4 × 10−4 | 40.2 | 0.005 | 2.9 × 10−4 | 332.2 | 0.99 |
| Sample | Temkin | Freundlich | Langmuir | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg g−1) | bt (J mol−1) | AT (mL mg−1) | R2 | KF (L mg−1) | n | R2 | KL (L mg−1) | qmax (mg g−1) | R2 | |
| MSN-SMZ | 215.4 | 37.5 | 1.1 | 0.85 | 33.5 | 1.4 | 0.88 | 0.08 | 442.0 | 0.90 |
| MSN-TETA-SMZ | 345.8 | 25.7 | 2.1 | 0.95 | 83.7 | 1.8 | 0.94 | 0.18 | 465.3 | 0.95 |
| Samples | k1 (h−1) | Amax (%) | Amax (µg mL−1) | R2 |
|---|---|---|---|---|
| MSN-SMZ | 136 | 69.8 | 171 | 0.98 |
| MSN-TETA-SMZ | 3.04 | 42.7 | 102 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carucci, C.; Scalas, N.; Porcheddu, A.; Piludu, M.; Monduzzi, M.; Salis, A. Adsorption and Release of Sulfamethizole from Mesoporous Silica Nanoparticles Functionalised with Triethylenetetramine. Int. J. Mol. Sci. 2021, 22, 7665. https://doi.org/10.3390/ijms22147665
Carucci C, Scalas N, Porcheddu A, Piludu M, Monduzzi M, Salis A. Adsorption and Release of Sulfamethizole from Mesoporous Silica Nanoparticles Functionalised with Triethylenetetramine. International Journal of Molecular Sciences. 2021; 22(14):7665. https://doi.org/10.3390/ijms22147665
Chicago/Turabian StyleCarucci, Cristina, Nicola Scalas, Andrea Porcheddu, Marco Piludu, Maura Monduzzi, and Andrea Salis. 2021. "Adsorption and Release of Sulfamethizole from Mesoporous Silica Nanoparticles Functionalised with Triethylenetetramine" International Journal of Molecular Sciences 22, no. 14: 7665. https://doi.org/10.3390/ijms22147665
APA StyleCarucci, C., Scalas, N., Porcheddu, A., Piludu, M., Monduzzi, M., & Salis, A. (2021). Adsorption and Release of Sulfamethizole from Mesoporous Silica Nanoparticles Functionalised with Triethylenetetramine. International Journal of Molecular Sciences, 22(14), 7665. https://doi.org/10.3390/ijms22147665