Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-Ceramisation: A New Way towards Implant Surface Modification
Abstract
:1. Introduction
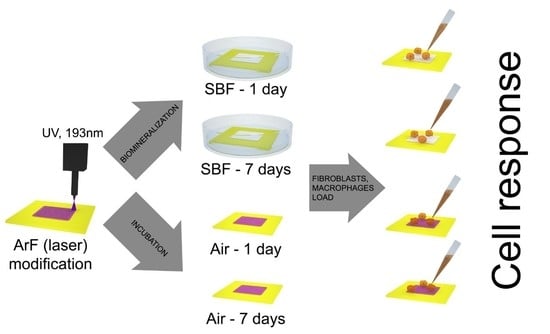
2. Results and Discussion
2.1. Physicochemical Analysis/Topography of PLLA Films
2.2. X-ray Photoelectron Spectroscopy Analysis
2.3. Cellular Response
3. Materials and Methods
3.1. Materials and Preparation of Samples
3.2. Laser Modification—PLLA Surface Activation
3.3. Incubation of PLLA in SBF
3.4. X-ray Photoelectron Microscopy (XPS)
3.5. Scanning Electron Microscopy (SEM)
3.6. Profilometry
3.7. Water Contact Angle Measurement
3.8. Cell Lines and Culture
3.9. Trypan Blue Exclusion Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable implant material review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Hazeli, K.; Niu, J.; Kontsos, A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Mechanical properties and biomineralization of multifunctional nanodiamond-PLLA composites for bone tissue engineering. Biomaterials 2012, 33, 5067–5075. [Google Scholar] [CrossRef]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Karimi, S.; Ghasemi, I.; Abbassi-Sourki, F. A study on the crystallization kinetics of PLLA in the presence of Graphene Oxide and PEG-grafted-Graphene Oxide: Effects on the nucleation and chain mobility. Compos. Part B Eng. 2019, 158, 302–310. [Google Scholar] [CrossRef]
- Chou, P.M.; Mariatti, M.; Zulkifli, A.; Sreekantan, S. Evaluation of the flexural properties and bioactivity of bioresorbable PLLA/PBSL/CNT and PLLA/PBSL/TiO2 nanocomposites. Compos. Part B Eng. 2012, 43, 1374–1381. [Google Scholar] [CrossRef]
- Kim, S.S.; Sun Park, M.; Jeon, O.; Yong Choi, C.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Nejati, E.; Firouzdor, V.; Eslaminejad, M.B.; Bagheri, F. Needle-like nano hydroxyapatite/poly(l-lactide acid) composite scaffold for bone tissue engineering application. Mater. Sci. Eng. C 2009, 29, 942–949. [Google Scholar] [CrossRef]
- Smieszek, A.; Marycz, K.; Szustakiewicz, K.; Kryszak, B.; Targonska, S.; Zawisza, K.; Watras, A.; Wiglusz, R.J. New approach to modification of poly (L-lactic acid) with nano-hydroxyapatite improving functionality of human adipose-derived stromal cells (hASCs) through increased viability and enhanced mitochondrial activity. Mater. Sci. Eng. C 2019, 98, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Wilberforce, S.I.J.; Finlayson, C.E.; Best, S.M.; Cameron, R.E. A comparative study of the thermal and dynamic mechanical behaviour of quenched and annealed bioresorbable poly-l-lactide/α-tricalcium phosphate nanocomposites. Acta Biomater. 2011, 7, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Szustakiewicz, K.; Stępak, B.; Antończak, A.J.; Maj, M.; Gazińska, M.; Kryszak, B.; Pigłowski, J. Femtosecond laser-induced modification of PLLA/hydroxypatite composite. Polym. Degrad. Stab. 2018, 149, 152–161. [Google Scholar] [CrossRef]
- Schugens, C.; Maquet, V.; Grandfils, C.; Jerome, R.; Teyssie, P. Biodegradable and macropororous polylactide implants for cell transplantation: 1. Preparation of macroporus polylactide supports by solid-liquid phase separation. Polymer 1996, 37, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Domura, R.; Sakai, R.; Okamoto, M.; Arakawa, S.; Ishiki, R.; Salick, M.R.; Turng, L.S. Fabrication of PLLA/HA composite scaffolds modified by DNA. Polymer 2015, 56, 73–81. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Gazińska, M.; Kryszak, B.; Grzymajło, M.; Pigłowski, J.; Wiglusz, R.J.; Okamoto, M. The influence of hydroxyapatite content on properties of poly(L-lactide)/hydroxyapatite porous scaffolds obtained using thermal induced phase separation technique. Eur. Polym. J. 2019, 113, 313–320. [Google Scholar] [CrossRef]
- Mansourizadeh, F.; Asadi, A.; Oryan, S.; Dodel, M.; Asghari-vostakolaei, M.; Therapy, C.; Cell, S. PLLA/HA Nano composite scaffolds for stem cell proliferation and differentiation in tissue engineering. Mol. Biol. Res. Commun. 2013, 2, 1–10. [Google Scholar]
- Prabhakaran, M.P.; Venugopal, J.; Ramakrishna, S. Electrospun nanostructured scaffolds for bone tissue engineering. Acta Biomater. 2009, 5, 2884–2893. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.K.; Converse, G.L.; Kane, R.J.; Yue, W. Hydroxyapatite-reinforced polymer biocomposites for synthetic bone substitutes. JOM 2008, 60, 38–45. [Google Scholar] [CrossRef]
- Wu, X.H.; Wu, Z.Y.; Su, J.C.; Yan, Y.G.; Yu, B.Q.; Wei, J.; Zhao, L.M. Nano-hydroxyapatite promotes self-assembly of honeycomb pores in poly(L-lactide) films through breath-figure method and MC3T3-E1 cell functions. RSC Adv. 2015, 5, 6607–6616. [Google Scholar] [CrossRef]
- Cao, L.; Weng, W.; Chen, X.; Ding, Y.; Yan, Y.; Li, H.; Zhao, H.; Shin, J.W.; Wei, J.; Ji, F.; et al. Development of degradable and bioactive composite as bone implants by incorporation of mesoporous bioglass into poly(L-lactide). Compos. Part B Eng. 2015, 77, 454–461. [Google Scholar] [CrossRef]
- Nie, L.; Chen, D.; Yang, Q.; Zou, P.; Feng, S.; Hu, H.; Suo, J. Hydroxyapatite/poly-l-lactide nanocomposites coating improves the adherence and proliferation of human bone mesenchymal stem cells on porous biphasic calcium phosphate scaffolds. Mater. Lett. 2013, 92, 25–28. [Google Scholar] [CrossRef]
- Ge, M.; Xue, L.; Nie, T.; Ma, H.; Zhang, J. The precision structural regulation of PLLA porous scaffold and its influence on the proliferation and differentiation of MC3T3-E1 cells. J. Biomater. Sci. Polym. Ed. 2016, 27, 1685–1697. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Iwata, T.; Doi, Y. Morphology and enzymatic degradation of poly(L-lactic acid) single crystals. Macromolecules 1998, 31, 2461–2467. [Google Scholar] [CrossRef]
- Farbod, K.; Nejadnik, M.R.; Jansen, J.A.; Leeuwenburgh, S.C.G. Interactions Between Inorganic and Organic Phases in Bone Tissue as a Source of Inspiration for Design of Novel Nanocomposites. Tissue Eng. Part B Rev. 2014, 20, 173–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006. [Google Scholar] [CrossRef]
- Zhang, F.; Chang, J.; Lu, J.; Ning, C. Surface modification of beta-tricalcium phosphate scaffolds with topological nanoapatite coatings. Mater. Sci. Eng. C 2008, 28, 1330–1339. [Google Scholar] [CrossRef]
- Peng, F.; Olson, J.R.; Shaw, M.T.; Wei, M. Influence of pretreatment on the surface characteristics of PLLA fibers and subsequent hydroxyapatite coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 220–229. [Google Scholar] [CrossRef]
- Silva, J.C.; Udangawa, R.N.; Chen, J.; Mancinelli, C.D.; Garrudo, F.F.F.; Mikael, P.E.; Cabral, J.M.S.; Ferreira, F.C.; Linhardt, R.J. Kartogenin-loaded coaxial PGS/PCL aligned nanofibers for cartilage tissue engineering. Mater. Sci. Eng. C 2020, 107. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E.; Kunkemoeller, B.; Wei, M. Evaluation of alkaline pre-treatment of PLLA fibers for biomimetic hydroxyapatite coating. Surf. Coatings Technol. 2014, 244, 23–28. [Google Scholar] [CrossRef]
- He, C.; Jin, X.; Ma, P.X. Calcium phosphate deposition rate, structure and osteoconductivity on electrospun poly(l-lactic acid) matrix using electrodeposition or simulated body fluid incubation. Acta Biomater. 2014, 10, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhu, L.; Liu, H.; Wen, W.; Li, H.; Zhou, C.; Luo, B. Biomineralization guided by polydopamine-modifed poly(L-lactide) fibrous membrane for promoted osteoconductive activity. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef]
- Szustakiewicz, K.; Kryszak, B.; Gazińska, M.; Chęcmanowski, J.; Grzymajło, M.; Stępak, B.; Antończak, A.J. The effect of selective mineralization of PLLA in simulated body fluid induced by ArF excimer laser irradiation: Tailored composites with potential in bone tissue engineering. Compos. Sci. Technol. 2020, 197, 1–9. [Google Scholar] [CrossRef]
- Koo, G.H.; Jang, J. Preparation of melting-free poly(lactic acid) by amorphous and crystal crosslinking under UV irradiation. J. Appl. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Belbachir, S.; Zaïri, F.; Ayoub, G.; Maschke, U.; Naït-Abdelaziz, M.; Gloaguen, J.M.; Benguediab, M.; Lefebvre, J.M. Modelling of photodegradation effect on elastic-viscoplastic behaviour of amorphous polylactic acid films. J. Mech. Phys. Solids 2010. [Google Scholar] [CrossRef]
- Santonja-Blasco, L.; Ribes-Greus, A.; Alamo, R.G. Comparative thermal, biological and photodegradation kinetics of polylactide and effect on crystallization rates. Polym. Degrad. Stab. 2013. [Google Scholar] [CrossRef]
- Tsuji, H.; Echizen, Y.; Nishimura, Y. Photodegradation of biodegradable polyesters: A comprehensive study on poly(l-lactide) and poly(ε-caprolactone). Polym. Degrad. Stab. 2006, 91, 1128–1137. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tsui, H.; Shimizu, K.; Sato, Y. Hydrolytic Degradation of Poly(L-lactic acid): Combined Effects of UV Treatment and Crystallization. J. Appl. Polym. Sci. 2012, 125, 2394–2406. [Google Scholar] [CrossRef]
- Slepička, P.; Michaljaničová, I.; Rimpelová, S.; Švorčík, V. Surface roughness in action—Cells in opposition. Mater. Sci. Eng. C 2017, 76, 818–826. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; Shive, M.S.; Colton, E.; Nakayama, Y.; Matsuda, T.; Anderson, J.M. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J. Biomed. Mater. Res. 2001, 55, 661–668. [Google Scholar] [CrossRef]
- Kryszak, B.; Szustakiewicz, K.; Stępak, B.; Gazińska, M.; Antończak, A.J. Structural, thermal and mechanical changes in poly(l-lactide)/hydroxyapatite composite extruded foils modified by CO2 laser irradiation. Eur. Polym. J. 2019, 114, 57–65. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Duran, H.; Üstün Alkan, F.; Ulkay, M.B.; Karakuş, S.; Aktaş, A.; Şişmanoğlu, T. Investigation of the in vitro cytotoxic effects and wound healing activity of ternary composite substance (hollow silica sphere/gum arabic/methylene blue). Int. J. Biol. Macromol. 2019, 121, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Pązik, R.; Zięcina, A.; Zachanowicz, E.; Małecka, M.; Poźniak, B.; Miller, J.; Śniadecki, Z.; Pierunek, N.; Idzikowski, B.; Mrõwczyńska, L.; et al. Synthesis, Structural Features, Cytotoxicity, and Magnetic Properties of Colloidal Ferrite Spinel Co1-xNixFe2O4 (0.1 ≤ x ≤ 0.9) Nanoparticles. Eur. J. Inorg. Chem. 2015, 2015, 4750–4760. [Google Scholar] [CrossRef]








| Sample | Sa [nm] | Sz [μm] | Ra [nm] | Rz [μm] |
|---|---|---|---|---|
| PLLA | 49±3 | 0.80±0.06 | 25±3 | 0.16±0.04 |
| PLLA_UV_1d | 36±7 | 0.45±0.07 | 21±3 | 0.15±0.02 |
| PLLA_UV_SBF_1d | 515±55 | 5.52±0.58 | 588±62 | 3.62±0.45 |
| PLLA_UV_7d | 38±4 | 0.83±0.15 | 30±3 | 0.15±0.03 |
| PLLA_UV_SBF_7d | 416±29 | 9.72±1.09 | 258±55 | 1.80±0.33 |
| C-C/C-H 285.0 eV | C-O-C=O 287.0 eV | O=C-O 289.1 eV | C-OH 286.4 eV | C=C 284.6 eV | O/C | Ca/C | |
|---|---|---|---|---|---|---|---|
| Theoretical ratio | 33.3 | 33.3 | 33.3 | - | - | 0.67 | - |
| PLLA | 63.5 | 16.3 | 16.3 | 3.9 | 0 | 0.25 | - |
| PLLA_UV_1d | 41.9 | 25.8 | 25.6 | 0.1 | 6.6 | 0.43 | - |
| PLLA_UV_7d | 39.1 | 29.0 | 26.2 | 0.6 | 5.1 | 0.48 | |
| PLLA_UV_SBF_1d | 28.9 | 31.8 | 31.0 | 4.1 | 4.2 | 0.53 | 0.005 |
| PLLA_UV_SBF_7d | 30.3 | 29.3 | 31.8 | 4.9 | 3.7 | 0.50 | 0.005 |
| Samples Description | Laser Treatment | Incubation in Air | Incubation in SBF |
|---|---|---|---|
| PLLA | − | - | - |
| PLLA_UV_1d | + | 1 day (24 h) | - |
| PLLA_UV_7d | + | 7 days (168 h) | - |
| PLLA_UV_SBF_1d | + | - | 1 day (24 h) |
| PLLA_UV_SBF_7d | + | - | 7 days (168 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szustakiewicz, K.; Kryszak, B.; Dzienny, P.; Poźniak, B.; Tikhomirov, M.; Hoppe, V.; Szymczyk-Ziółkowska, P.; Tylus, W.; Grzymajło, M.; Gadomska-Gajadhur, A.; et al. Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-Ceramisation: A New Way towards Implant Surface Modification. Int. J. Mol. Sci. 2021, 22, 8436. https://doi.org/10.3390/ijms22168436
Szustakiewicz K, Kryszak B, Dzienny P, Poźniak B, Tikhomirov M, Hoppe V, Szymczyk-Ziółkowska P, Tylus W, Grzymajło M, Gadomska-Gajadhur A, et al. Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-Ceramisation: A New Way towards Implant Surface Modification. International Journal of Molecular Sciences. 2021; 22(16):8436. https://doi.org/10.3390/ijms22168436
Chicago/Turabian StyleSzustakiewicz, Konrad, Bartłomiej Kryszak, Paulina Dzienny, Błażej Poźniak, Marta Tikhomirov, Viktoria Hoppe, Patrycja Szymczyk-Ziółkowska, Włodzimierz Tylus, Michał Grzymajło, Agnieszka Gadomska-Gajadhur, and et al. 2021. "Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-Ceramisation: A New Way towards Implant Surface Modification" International Journal of Molecular Sciences 22, no. 16: 8436. https://doi.org/10.3390/ijms22168436
APA StyleSzustakiewicz, K., Kryszak, B., Dzienny, P., Poźniak, B., Tikhomirov, M., Hoppe, V., Szymczyk-Ziółkowska, P., Tylus, W., Grzymajło, M., Gadomska-Gajadhur, A., & Antończak, A. J. (2021). Cytotoxicity Study of UV-Laser-Irradiated PLLA Surfaces Subjected to Bio-Ceramisation: A New Way towards Implant Surface Modification. International Journal of Molecular Sciences, 22(16), 8436. https://doi.org/10.3390/ijms22168436