TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation
Abstract
:1. Introduction
2. TRPM8 Distribution and Pathological Implication
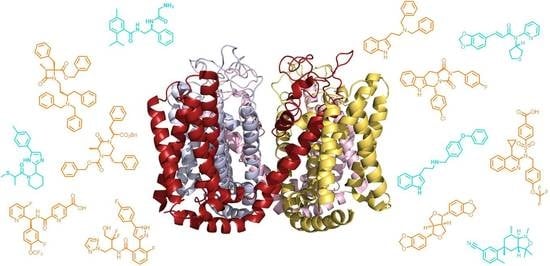
3. Insights into TRPM8 Structure

3.1. Structures of TRPM8 in Complex with Agonists
3.2. Structures of TRPM8 in Complex with Antagonists
4. TRPM8 Agonists
| Compd. | Structure | TRPM8 EC50 a (µM) | Application | Ref. |
|---|---|---|---|---|
| 4 |  | 0.0019 | Potential treatment for obesity, diabetes, dyslipidemia, irritable bowel syndrome, pain, personal care products or food additive | [127] |
| 7 |  | 6 | Personal care products. In tooth paste, rapid onset and long duration of cooling sensation | [128] |
| 10 |  | ≤0.05 | Ranked as “strong” regarding maximal cooling, and “medium” after 1 h | [130] |
| 15 |  | <0.05 | Ranked as “extreme” regarding maximal cooling, and “medium” after 1 h | [131,132] |
| 16 |  | 0.9 | Sensory and mask discomfort Gastrointestinal tract disorders Pain Dry eye disease | [136,137] |
| 17 |  | 40 b | Pain | [139,140] |
5. TRPM8 Antagonists
| Compd. | Structure | IC50 (μM) | In Vivo Inhibitory Activity | Ref. | |
|---|---|---|---|---|---|
| Ca2+ Assay | Patch-Clamp | ||||
| 18 |  | 9.8 | _ | Antitumoral | [144] |
| 19 |  | 9.7 | _ | _ | [145] |
| 22 SKP-5714 |  | 0.025 | _ | Cold sensibility (WDS) Bladder overactivity | [149,150] |
| 24 |  | 0.21 | _ | Cold sensibility (WDS) | [154] |
| 28 (AMG333) |  | 0.013 | _ | Cold sensibility (WDS) | [161] |
| 29 |  | 1.8 | 0.70 | _ | [163] |
| 31 |  | 3.2 | 0.37 | Skin hypersensitivity Neuropathic pain Cold sensibility (WDS) Reducted temperature | [139,140] |
| 34 |  | 0.04 | 0.0002 | Cold sensibility (WDS) Oxaliplatin-induced neuropathy | [164] |
| 37 |  | 0.06 | 0.004 | Cold sensibility (WDS) Improved oxaliplatin-induced neuropathy | [165] |
| 40 |  | 0.4 | 0.8 | Improved oxaliplatin-induced neuropathy Antitumor activity | [168] |
| 41 |  | 0.16 | _ | _ | [168] |
| 42 |  | 0.02 | 0.05 | _ | [169] |
| 43 (M8-B) |  | 64.3 | _ | Decreased body temperature Epilepsy | [170] |
6. Clinical Trials with TRPM8 Modulators
| Compound | Function | Route of Administration | Indication | Clinical Phase | Clinical Trial Number | Ref. |
|---|---|---|---|---|---|---|
| Menthol (1) | Agonist | Topical | Pain, after photodynamic therapy | 4 | NCT02984072 | _ |
| Menthol (1) | Agonist | Topical | Pain, after TRPA1 agonist | NA | NCT02653703 | [171] |
| Menthol (1) (Biofreeze) | Agonist | Topical | Knee osteoarthritis Mechanical/Neck pain | 2 Withdrawn NA | NCT04351594 NCT01565070 NCT03012503 | [172] _ _ |
| Menthol (1) | Agonist | Topical | Chemotherapy-induced peripheral neuropathy | 2 | NCT01855607 | [173] |
| Menthol (1) | Agonist | Topical | Carpal tunnel syndrome | NA | NCT01716767 | [174] |
| Menthol (1) + manitol | Agonist | Topical | Diabetic peripheral neuropathy | 1–2 | NCT02728687 | _ |
| Menthol (1) (STOPAIN®) | Agonist | Topical | Migraine | NA | NCT01687101 | [175,176] |
| Menthol (1) | Agonist | Oral | Oropharyngeal dysphagia | 2 | NCT03050957 | _ |
| Menthol (1) | Agonist | Oral | Hypertension | 2 | NCT01408446 | [177] |
| Menthoxypro- panediol | Agonist | Topical | Pruritus Atopic dermatitis | NA | NCT03610386 | [178] |
| Cryosim-1 | Agonist | Topical | Itch | NA | ND | [179] |
| Cryosim-3 (16) | Agonist | Topical | Dry eye disease | NA | ND | [136] |
| D-3263 | Agonist | Oral | Cancer | 1 | NCT00839631 | [181] |
| Cannabidivarin | Antagonist | Autism Epilepsy | 2 2 | NCT03849456 NCT02369471 | [183] _ | |
| PF-05105679 | Antagonist | Oral | Pain | 1 | NCT01393652 | [184] |
| AMG 333 (28) | Antagonist | Oral | Migraine | 1 | NCT01953341 | [161] |
| Diagnosys test | _ | _ | Cold, dry air | NA | NCT04286542 | _ |
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleig, A.; Penner, R. The TRPM ion channel subfamily: Molecular, biophysical and functional features. Trends Pharmacol. Sci. 2004, 25, 633–639. [Google Scholar] [CrossRef]
- Huang, Y.; Fliegert, R.; Guse, A.H.; Lu, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85, 102111. [Google Scholar] [CrossRef]
- Fonfria, E.; Murdock, P.R.; Cusdin, F.S.; Benham, C.D.; Kelsell, R.E.; McNulty, S. Tissue distribution profiles of the human TRPM cation channel family. J. Recept. Signal Transduct. 2006, 26, 159–178. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [Green Version]
- García-Ávila, M.; Islas, L.D. What is new about mild temperature sensing? A review of recent findings. Temperature 2019, 6, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, K.; Voets, T.; Vriens, J. TRPM3 in temperature sensing and beyond. Temperature 2015, 2, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Liman, E.R. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc. Natl. Acad. Sci. USA 2003, 100, 15160–15165. [Google Scholar] [CrossRef] [Green Version]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef] [Green Version]
- Simon, F.; Varela, D.; Cabello-Verrugio, C. Oxidative stress-modulated TRPM ion channels in cell dysfunction and pathological conditions in humans. Cell. Signal. 2013, 25, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2007, 1772, 813–821. [Google Scholar] [CrossRef] [Green Version]
- McNulty, S.; Fonfria, E. The role of TRPM channels in cell death. Pflug. Arch. Eur. J. Physiol. 2005, 451, 235–242. [Google Scholar] [CrossRef]
- Earley, S.; Waldron, B.J.; Brayden, J.E. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ. Res. 2004, 95, 922–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zholos, A.; Johnson, C.; Burdyga, T.; Melanaphy, D. TRPM channels in the vasculature. In Transient Receptor Potential Channels; Islam, M.S., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 707–729. ISBN 978-94-007-0265-3. [Google Scholar]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Tsavaler, L.; Shapero, M.H.; Morkowski, S.; Laus, R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001, 61, 3760–3769. [Google Scholar]
- Dhaka, A.; Earley, T.J.; Watson, J.; Patapoutian, A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 2008, 28, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Denda, M.; Tsutsumi, M.; Denda, S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: Role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp. Dermatol. 2010, 19, 791–795. [Google Scholar] [CrossRef]
- Pan, Y.; Thapa, D.; Baldissera, L.; Argunhan, F.; Aubdool, A.A.; Brain, S.D. Relevance of TRPA1 and TRPM8 channels as vascular sensors of cold in the cutaneous microvasculature. Pflug. Arch. Eur. J. Physiol. 2018, 470, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Keh, S.M.; Facer, P.; Yehia, A.; Sandhu, G.; Saleh, H.A.; Anand, P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology 2011, 49, 11. [Google Scholar] [CrossRef] [Green Version]
- El Karim, I.A.; Linden, G.J.; Curtis, T.M.; About, I.; McGahon, M.K.; Irwin, C.R.; Killough, S.A.; Lundy, F.T. Human dental pulp fibroblasts express the “cold-sensing” transient receptor potential channels TRPA1 and TRPM8. J. Endod. 2011, 37, 473–478. [Google Scholar] [CrossRef]
- Tazawa, K.; Ikeda, H.; Kawashima, N.; Okiji, T. Transient receptor potential melastatin (TRPM) 8 is expressed in freshly isolated native human odontoblasts. Arch. Oral Biol. 2017, 75, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Lu, H.H.; Cheng, L.H.; Chu, Y.H.; Lee, F.P.; Wu, C.C.; Wang, H.W. Identification of the cold receptor TRPM8 in the nasal mucosa. Am. J. Rhinol. Allergy 2015, 29, e112–e116. [Google Scholar] [CrossRef] [PubMed]
- Abe, J.; Hosokawa, H.; Okazawa, M.; Kandachi, M.; Sawada, Y.; Yamanaka, K.; Matsumura, K.; Kobayashi, S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Mol. Brain Res. 2005, 136, 91–98. [Google Scholar] [CrossRef]
- Parra, A.; Madrid, R.; Echevarria, D.; Del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat. Med. 2010, 16, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.J.; Santos, S.; Nagatomi, J.; Hayashi, Y.; Minnery, B.S.; Xavier, M.; Patel, A.S.; Nelson, J.B.; Futrell, W.J.; Yoshimura, N.; et al. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. J. Urol. 2004, 172, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hill, W.G.; Apodaca, G.; Zeidel, M.L. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am. J. Physiol. 2011, 300, F49–F59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanneste, M.; Segal, A.; Voets, T.; Everaerts, W. Transient receptor potential channels in sensory mechanisms of the lower urinary tract. Nat. Rev. Urol. 2021, 18, 139–159. [Google Scholar] [CrossRef] [PubMed]
- De Blas, G.A.; Darszon, A.; Ocampo, A.Y.; Serrano, C.J.; Castellano, L.E.; Hernandez-Gonzalez, E.O.; Chirinos, M.; Larrea, F.; Beltran, C.; Trevino, C.L. TRPM8, a versatile channel in human sperm. PLoS ONE 2009, 4, e6095. [Google Scholar] [CrossRef] [Green Version]
- Amato, A.; Terzo, S.; Lentini, L.; Marchesa, P.; Mule, F. TRPM8 Channel activation reduces the spontaneous contractions in human distal colon. Int. J. Mol. Sci. 2020, 21, 5403. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, A.S.; Shadid, M.; Yost, G.S.; Reilly, C.A. Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am. J. Respir. Cell Mol. Biol. 2008, 39, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, Q.; Yang, G.; Kolosov, V.P.; Perelman, J.M.; Zhou, X.D. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J. Allergy Clin. Immunol. 2011, 128, 626–634.e5. [Google Scholar] [CrossRef]
- Khalil, M.; Babes, A.; Lakra, R.; Försch, S.; Reeh, P.W.; Wirtz, S.; Becker, C.; Neurath, M.F.; Engel, M.A. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol. 2016, 9, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, E.; Peiris, M.; Peiris, M.; King, H.W.; Wing, E.S.; Lindsay, J.O.; Blackshaw, L.A.; Stagg, A.J. P016 Constitutive activity of the cation channel TRPM8 regulates monocyte to macrophage transition in humans to control intestinal inflammation. J. Crohn’s Colitis 2019, 13, S094. [Google Scholar] [CrossRef]
- Ordás, P.; Hernandez-Ortego, P.; Vara, H.; Fernandez-Pena, C.; Reimundez, A.; Morenilla-Palao, C.; Guadano-Ferraz, A.; Gomis, A.; Hoon, M.; Viana, F.; et al. Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits. J. Comp. Neurol. 2021, 529, 234–256. [Google Scholar] [CrossRef]
- Bidaux, G.; Gordienko, D.; Shapovalov, G.; Farfariello, V.; Borowiec, A.-S.; Iamshanova, O.; Lemonnier, L.; Gueguinou, M.; Guibon, R.; Fromont, G.; et al. 4TM-TRPM8 channels are new gatekeepers of the ER-mitochondria Ca2+ transfer. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.A.; Skryma, R.; Bidaux, G.; Magleby, K.L.; Scholfeld, C.N.; McGeown, J.G.; Prevarskaya, N.; Zholos, A.V. Voltage-and cold-dependent gating of single TRPM8 ion channels. J. Gen. Physiol. 2011, 137, 173–195. [Google Scholar] [CrossRef] [Green Version]
- Raddatz, N.; Castillo, J.P.; Gonzalez, C.; Alvarez, O.; Latorre, R. Temperature and voltage coupling to channel opening in transient receptor potential melastatin 8 (TRPM8). J. Biol. Chem. 2014, 289, 35438–35454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. Nat. Commun. 2015, 6, 7150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parra, A.; Gonzalez-Gonzalez, O.; Gallar, J.; Belmonte, C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain 2014, 155, 1481–1491. [Google Scholar] [CrossRef] [Green Version]
- Blanquart, S.; Borowiec, A.; Delcourt, P.; Figeac, M.; Emerling, C.A.; Meseguer, A.S.; Roudbaraki, M.; Prevarskaya, N.; Bidaux, G. Evolution of the human cold/menthol receptor, TRPM8. Mol. Phylogenet. Evol. 2019, 136, 104–118. [Google Scholar] [CrossRef]
- Bidaux, G.; Beck, B.; Zholos, A.; Gordienko, D.; Lemonnier, L.; Flourakis, M.; Roudbaraki, M.; Borowiec, A.S.; Fernández, J.; Delcourt, P.; et al. Regulation of activity of transient receptor potential melastatin 8 (TRPM8) channel by its short isoforms. J. Biol. Chem. 2012, 287, 2948–2962. [Google Scholar] [CrossRef] [Green Version]
- Pertusa, M.; Madrid, R.; Morenilla-Palao, C.; Belmonte, C.; Viana, F. N-glycosylation of TRPM8 ion channels modulates temperature sensitivity of cold thermoreceptor neurons. J. Biol. Chem. 2012, 287, 18218–18229. [Google Scholar] [CrossRef] [Green Version]
- Pertusa, M.; González, A.; Hardy, P.; Madrid, R.; Viana, F. Bidirectional modulation of thermal and chemical sensitivity of TRPM8 channels by the initial region of the N-terminal domain. J. Biol. Chem. 2014, 289, 21828–21843. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Yudin, Y.; Bikard, Y.; Chen, W.; Liu, T.; Li, H.; Jendrossek, D.; Cohen, A.; Pavlov, E.; Rohacs, T.; et al. Polyester modification of the mammalian trpm8 channel protein: Implications for structure and function. Cell Rep. 2013, 4, 302–315. [Google Scholar] [CrossRef] [Green Version]
- Rohács, T.; Lopes, C.M.B.; Michailidis, I.; Logothetis, D.E. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005, 8, 626–634. [Google Scholar] [CrossRef]
- Liu, B.; Qin, F. Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 2005, 25, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Yudin, Y.; Lukacs, V.; Cao, C.; Rohacs, T. Decrease in phosphatidylinositol 4,5-bisphosphate levels mediates desensitization of the cold sensor TRPM8 channels. J. Physiol. 2011, 589, 6007–6027. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kim, A.; Masuch, T.; Park, K.; Weng, H.; Wetzel, C.; Dong, X. Pirt functions as an endogenous regulator of TRPM8. Nat. Commun. 2013, 4, 2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X. Direct Gαq Gating is the sole mechanism for TRPM8 inhibition caused by bradykinin receptor activation. Cell Rep. 2019, 27, 3672–3683.e4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mak, S.; Li, L.; Parra, A.; Denlinger, B.; Belmonte, C.; McNaughton, P.A. Direct inhibition of the cold-Activated TRPM8 ion channel by Gα q. Nat. Cell Biol. 2012, 14, 850–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Rohacs, T. Regulation of the cold-sensing TRPM8 channels by phosphoinositides and Gq-coupled receptors. Channels 2020, 14, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Manolache, A.; Selescu, T.; Maier, G.L.; Neacsu, C.; Babes, A.; Mentel, M.; Ionescu, A.E.; Szedlacsek, S.E. Regulation of TRPM8 channel activity by Src-mediated tyrosine phosphorylation. J. Cell. Physiol. 2020, 235, 5192–5203. [Google Scholar] [CrossRef] [PubMed]
- Gkika, D.; Lolignier, S.; Grolez, G.P.; Bavencoffe, A.; Shapovalov, G.; Gordienko, D.; Kondratskyi, A.; Meleine, M.; Prival, L.; Chapuy, E.; et al. Testosterone-androgen receptor: The steroid link inhibiting TRPM8-mediated cold sensitivity. FASEB J. 2020, 34, 7483–7499. [Google Scholar] [CrossRef] [Green Version]
- Madrid, R.; Pertusa, M. Intimacies and physiological role of the polymodal cold-sensitive ion channel TRPM8. Curr. Tops. Membr. 2014, 74, 293–324. [Google Scholar] [CrossRef]
- Gavva, N.R.; Davis, C.; Lehto, S.G.; Rao, S.; Wang, W.; Zhu, D.X.D. Transient receptor potential melastatin 8 (TRPM8) channels are involved in body temperature regulation. Mol. Pain 2012, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- McCoy, D.D.; Zhou, L.; Nguyen, A.K.; Watts, A.G.; Donovan, C.M.; McKemy, D.D. Enhanced insulin clearance in mice lacking TRPM8 channels. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, G.M.; Orta, G.; Reddy, T.; Koppers, A.J.; Martinez-Lopez, P.; de la Vega-Beltran, J.L.; Lo, J.C.Y.; Veldhuis, N.; Jamsai, D.; McIntyre, P.; et al. Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc. Nat. Acad. Sci. USA 2011, 108, 7034–7039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaraz, L.; Manenschijn, J.-A.; de la Peña, E.; Viana, F. TRPM8. In Mammalian Transient Receptor Potential (TRP) Cation Channels: Volume I; Nilius, B., Flockerzi, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 547–579. ISBN 978-3-642-54215-2. [Google Scholar]
- Knowlton, W.M.; Palkar, R.; Lippoldt, E.K.; McCoy, D.D.; Baluch, F.; Chen, J.; McKemy, D.D. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 2013, 33, 2837–2848. [Google Scholar] [CrossRef] [Green Version]
- Kovács, I.; Luna, C.; Quirce, S.; Mizerska, K.; Callejo, G.; Riestra, A.; Fernández-Sánchez, L.; Meseguer, V.M.; Cuenca, N.; Merayo-Lloves, J.; et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain 2016, 157, 399–417. [Google Scholar] [CrossRef]
- Yang, J.M.; Wei, E.T.; Kim, S.J.; Yoon, K.C. TRPM8 Channels and Dry Eye. Pharmaceuticals 2018, 11, 125. [Google Scholar] [CrossRef] [Green Version]
- Pina, R.; Ugarte, G.; Campos, M.; Inigo-Portugues, A.; Olivares, E.; Orio, P.; Belmonte, C.; Bacigalupo, J.; Madrid, R. Role of trpm8 channels in altered cold sensitivity of corneal primary sensory neurons induced by axonal damage. J. Neurosci. 2019, 39, 8177–8192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dussor, G.; Cao, Y.Q. TRPM8 and migraine. Headache 2016, 56, 1406–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descoeur, J.; Pereira, V.; Pizzoccaro, A.; Francois, A.; Ling, B.; Maffre, V.; Couette, B.; Busserolles, J.; Courteix, C.; Noel, J.; et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 2011, 3, 266–278. [Google Scholar] [CrossRef]
- Gauchan, P.; Andoh, T.; Kato, A.; Kuraishi, Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 2009, 458, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, M.; Ling, J.; Tan, W.; Gu, J.G. TRPM8 mechanism of cold allodynia after chronic nerve injury. J. Neurosci. 2007, 27, 13680–13690. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Hao, J.; Wiesenfeld-Hallin, Z.; Xu, X.J. Activation of TRPM8 cold receptor triggers allodynia-like behavior in spinally injured rats. Scand. J. Pain 2013, 4, 33–37. [Google Scholar] [CrossRef]
- Gong, K.; Jasmin, L. Sustained morphine administration induces TRPM8-dependent cold hyperalgesia. J. Pain 2017, 18, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.H.; Chen, S.P.; Fann, C.S.J.; Wang, S.J.; Wang, Y.F. TRPM8 genetic variant is associated with chronic migraine and allodynia. J. Headache Pain 2019, 20, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todorovic, S.M. Is diabetic nerve pain caused by dysregulated ion channels in sensory neurons? Diabetes 2015, 64, 3987–3989. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, M.; Zochodne, D.W. Diabetic neuropathy and the sensory neuron: New aspects of pathogenesis and their treatment implications. J. Diabetes Investig. 2018, 9, 1239–1254. [Google Scholar] [CrossRef]
- Moisset, X.; Ouchchane, L.; Guy, N.; Bayle, D.J.; Dallel, R.; Clavelou, P. Migraine headaches and pain with neuropathic characteristics: Comorbid conditions in patients with multiple sclerosis. Pain 2013, 154, 2691–2699. [Google Scholar] [CrossRef] [Green Version]
- Truini, A.; Barbanti, P.; Pozzilli, C.; Cruccu, G. A mechanism-based classification of pain in multiple sclerosis. J. Neurol. 2013, 260, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoya, T.; Matsumoto, K.; Tashima, K.; Nakamura, H.; Fujino, H.; Murayama, T.; Horie, S. TRPM8 has a key role in experimental colitis-induced visceral hyperalgesia in mice. Neurogastroenterol. Motil. 2014, 26, 1112–1121. [Google Scholar] [CrossRef]
- De Jong, P.R.; Takahashi, N.; Peiris, M.; Bertin, S.; Lee, J.; Gareau, M.G.; Paniagua, A.; Harris, A.R.; Herdman, D.S.; Corr, M.; et al. TRPM8 on mucosal sensory nerves regulates colitogenic responses by innate immune cells via CGRP. Mucosal Immunol. 2015, 8, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henström, M.; Hadizadeh, F.; Beyder, A.; Bonfiglio, F.; Zheng, T.; Assadi, G.; Rafter, J.; Bujanda, L.; Agreus, L.; Andreasson, A.; et al. TRPM8 polymorphisms associated with increased risk of IBS-C and IBS-M. Gut 2017, 66, 1725–1727. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.Z.; Unno, S.; Kitagawa, J.; Ando, H.; Masuda, Y. Activation of TRPV1 and TRPM8 channels in the larynx and associated laryngopharyngeal regions facilitates the swallowing reflex. Int. J. Mol. Sci. 2018, 19, 4113. [Google Scholar] [CrossRef] [Green Version]
- Buday, T.; Brozmanova, M.; Biringerova, Z.; Gavliakova, S.; Poliacek, I.; Calkovsky, V.; Shetthalli, M.V.; Plevkova, J. Modulation of cough response by sensory inputs from the nose-role of trigeminal TRPA1 versus TRPM8 channels. Cough 2012, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonvini, S.J.; Belvisi, M.G. Cough and airway disease: The role of ion channels. Pulm. Pharmacol. Ther. 2017, 47, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, Y.S.; Kim, H.I.; Park, J.Y.; Park, S.H.; Hwang, Y.I.; Jang, S.H.; Jung, K.S.; Park, H.S.; Park, C.S. Activation of transient receptor potential melastatin family member 8 (trpm8) receptors induces proinflammatory cytokine expressions in bronchial epithelial cells. Allergy Asthma Immunol. Res. 2020, 12, 684–700. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Yang, G.; Zhou, X. Transient Receptor Potential Melastatin 8 (TRPM8)-based mechanisms underlie both the cold temperature-induced inflammatory reactions and the synergistic effect of cigarette smoke in human bronchial epithelial (16HBE) cells. Front. Physiol. 2019, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Ni, M.; Zhang, J.M.; Li, D.J.; Shen, F.M. TRPM8 downregulation by angiotensin II in vascular smooth muscle cells is involved in hypertension. Mol. Med. Rep. 2017, 15, 1900–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.; Huang, H.; Sun, D.; Lee, C. TRPM8 and RAAS-mediated hypertension is critical for cold- induced immunosuppression in mice. Oncotarget 2018, 9, 12781–12795. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Wang, B.; Lin, S.; Zhang, H.; Li, Y.; Wei, X.; Cui, Y.; Wei, X.; Lu, Z.; Gao, P.; et al. Activation of Transient Receptor Potential Melastatin Subtype 8 attenuates cold-induced hypertension through ameliorating vascular mitochondrial dysfunction. J. Am. Hear. Assoc. 2017, 6, e005495. [Google Scholar] [CrossRef]
- Yee, N.S. Roles of TRPM8 ion channels in cancer: Proliferation, survival, and invasion. Cancers 2015, 7, 2134–2146. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, S.; Jia, Z.; Zhao, W.; Zhou, C.; Zhang, R. Transient Receptor Potential Melastatin 8 (TRPM8) channel regulates proliferation and migration of breast cancer cells by activating the AMPK-ULK1 pathway to enhance basal autophagy. Front. Oncol. 2020, 10, 573127. [Google Scholar] [CrossRef]
- Yuan, L.; Ju, L. TRPM8 inhibition regulates the proliferation, migration and ROS metabolism of bladder cancer. Onco. Targets Ther. 2020, 13, 8825–8835. [Google Scholar] [CrossRef]
- Lan, X.; Zhao, J.; Song, C.; Yuan, Q. TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. Biosci. Rep. 2019, 39, BSR20191878. [Google Scholar] [CrossRef] [Green Version]
- Du, G.; Li, J.; Liu, W.; Liu, Y.; Zhao, B. The combination of TRPM8 and TRPA1 expression causes an invasive phenotype in lung cancer. Tumour Biol. 2014, 35, 1251–1261. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Meng, Z.; Cao, H.; Zhu, G.; Liu, T.; Wang, X. Knockdown of TRPM8 suppresses cancer malignancy and enhances epirubicin-induced apoptosis in human osteosarcoma cells. Int. J. Biol. Sci. 2014, 10, 90–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wu, H.; Wei, Z.; Wang, X.; Shen, P.; Wang, S.; Wang, A.; Chen, W.; Lu, Y. TRPM8: A potential target for cancer treatment. J. Cancer Res. Clin. Oncol. 2016, 142, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Perez de Vega, M.J.; Gomez-Monterrey, I.; Ferrer-Montiel, A.; Gonzalez-Muniz, R. Transient Receptor Potential Melastatin 8 channel (TRPM8) modulation: Cool entryway for treating pain and cancer. J. Med. Chem. 2016, 59, 10006–10029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Muniz, R.; Bonache, M.A.; Martin-Escura, C.; Gomez-Monterrey, I. Recent progress in TRPM8 modulation: An update. Int. J. Mol. Sci. 2019, 20, 2618. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef]
- Liu, J.-J.; Li, L.-Z.; Xu, P. Upregulation of TRPM8 can promote the colon cancer liver metastasis through mediating Akt/GSK-3 signal pathway. Biotechnol. Appl. Biochem. 2021. [Google Scholar] [CrossRef]
- Lunardi, A.; Fortuna, N.; Genovesi, S.; Alaimo, A.; Barbareschi, M.; Carbone, F.G.; Morelli, L.; Brunelli, M. TRPM8 protein expression in hormone naive local and lymph node metastatic prostate cancer. Pathologica 2021, 113, 95–101. [Google Scholar] [CrossRef]
- Hemida, A.S.; Hammam, M.A.; Heriz, N.A.E.M.; Shehata, W.A. Expression of Transient Receptor Potential Channel of Melastatin number 8 (TRPM8) in non-melanoma skin cancer: A clinical and immunohistochemical study. J. Immunoass. Immunochem. 2021. [Google Scholar] [CrossRef]
- Chinigo, G.; Chinigo, G.; Castel, H.; Chever, O.; Castel, H.; Chever, O.; Gkika, D.; Gkika, D. TRP Channels in brain tumors. Front. Cell Dev. Biol. 2021, 9, 617801. [Google Scholar] [CrossRef]
- Zeng, J.; Wu, Y.; Mungur, R.; Pan, J.; Zhu, Y.; Zhan, R.; Zhuang, S.; Hua, S.; Qin, L. Identification of the role of TRPM8 in glioblastoma and its effect on proliferation, apoptosis and invasion of the U251 human glioblastoma cell line. Oncol. Rep. 2019, 42, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Huang, Y.; Huang, M.; Zhang, M.; Chen, Y.; Huang, Z.; Cui, M.; Liang, W.; Liu, S.; Cai, X. Significance of TRPM8 protein expression in lung adenocarcinoma. Chin. J. Pathophysiol. 2020, 36, 1680–1689. [Google Scholar] [CrossRef]
- Rimola, V.; Osthues, T.; Koenigs, V.; Geisslinger, G.; Sisignano, M. Oxaliplatin causes transient changes in TRPM8 channel activity. Int. J. Mol. Sci. 2021, 22, 4962. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Zhou, F.; Zhang, P. Increased Transient Receptor Potential Melastatin 8 expression in the development of bladder pain in patients with interstitial cystitis/bladder pain syndrome. Urology 2020, 146, e1–e301. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zhao, M.; Ding, N.; Ge, N.; Wang, S.; Zhang, X.; Daugherty, S.L.; Beckel, J.M. Activation of TRPM8 channel inhibits contraction of the isolated human ureter. Neurourol. Urodyn. 2021. [Google Scholar] [CrossRef]
- Uslusoy, F.; Nazrolu, M.; Ertilav, K. Regeneration of mechanical sciatic nerve injury is affected by cold and heat exposure: Involvements of the TRPM2 and TRPM8 channels. Int. J. Burn. Trauma 2020, 10, 279–295. [Google Scholar] [PubMed]
- Fozzato, S.; Baranzini, N.; Bossi, E.; Cinquetti, R.; Grimaldi, A.; Campomenosi, P.; Surace, M.F.; Bossi, E.; Surace, M.F. TRPV4 and TRPM8 as putative targets for chronic low back pain alleviation. Pflug. Arch. 2021, 473, 151–165. [Google Scholar] [CrossRef]
- Gualdani, R.; Yuan, J.-H.; Effraim, P.R.; Di, S.G.; Truini, A.; Cruccu, G.; Dib-Hajj, S.D.; Gailly, P.; Waxman, S.G. Trigeminal neuralgia TRPM8 mutation: Enhanced activation, basal [Ca(2+)]i and menthol response. Neurol. Genet. 2021, 7, e550. [Google Scholar] [CrossRef] [PubMed]
- Weyer-Menkhoff, I.; Lotsch, J.; Pinter, A.; Schlierbach, H.; Schanzer, A.; Lotsch, J. Epidermal expression of human TRPM8, but not of TRPA1 ion channels, is associated with sensory responses to local skin cooling. Pain 2019, 160, 2699–2709. [Google Scholar] [CrossRef]
- Schecterson, L.C.; Pazevic, A.A.; Yang, R.; Matulef, K.; Gordon, S.E. TRPV1, TRPA1, and TRPM8 are expressed in axon terminals in the cornea: TRPV1 axons contain CGRP and secretogranin II; TRPA1 axons contain secretogranin 3. Mol. Vis. 2020, 26, 576–587. [Google Scholar] [PubMed]
- Lee, P.R.; Lee, J.Y.; Kim, H.B.; Oh, S.B.; Lee, J.H.; Oh, S.B. TRPM8 mediates hyperosmotic stimuli-induced nociception in dental afferents. J. Dent. Res. 2020, 99, 107–114. [Google Scholar] [CrossRef]
- Typolt, O.; Filingeri, D. Evidence for the involvement of peripheral cold-sensitive TRPM8 channels in human cutaneous hygrosensation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R579–R589. [Google Scholar] [CrossRef]
- Acharya, T.K.; Tiwari, A.; Majhi, R.K.; Goswami, C. TRPM8 channel augments T-cell activation and proliferation. Cell Biol. Int. 2021, 45, 198–210. [Google Scholar] [CrossRef]
- Fedinec, A.L.; Liu, J.; Zhang, R.; Harsono, M.; Pourcyrous, M.; Parfenova, H. The cold receptor TRPM8 activation leads to attenuation of endothelium-dependent cerebral vascular functions during head cooling. J. Cereb. Blood Flow Metab. 2021, 271678X211018035. [Google Scholar] [CrossRef]
- Cornejo, V.H.; Gonzalez, C.; Campos, M.; Vargas-Saturno, L.; de los Ángeles Juricic, M.; Miserey-Lenkei, S.; Pertusa, M.; Madrid, R.; Couve, A. Non-conventional axonal organelles control TRPM8 ion channel trafficking and peripheral cold sensing. Cell Rep. 2020, 30, 4505–4517.e5. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wu, M.; Zubcevic, L.; Borschel, W.F.; Lander, G.C.; Lee, S.Y. Structure of the cold- And menthol-sensing ion channel TRPM8. Science 2018, 359, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.; Lee, S.Y. Current view of ligand and lipid recognition by the menthol receptor TRPM8. Trends Biochem. Sci. 2020, 45, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Dubin, A.E.; Petrus, M.J.; Orth, A.; Mathur, J.; Hwang, S.W.; Patapoutian, A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 2006, 9, 493–500. [Google Scholar] [CrossRef]
- Beccari, A.R.; Gemei, M.; Monte, M.L.; Menegatti, N.; Fanton, M.; Pedretti, A.; Bovolenta, S.; Nucci, C.; Molteni, A.; Rossignoli, A.; et al. Novel selective, potent naphthyl TRPM8 antagonists identified through a combined ligand-and structure-based virtual screening approach. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Voets, T.; Owsianik, G.; Janssens, A.; Talavera, K.; Nilius, B. TRPM8 voltage sensor mutants reveal a mechanism for integrating thermal and chemical stimuli. Nat. Chem. Biol. 2007, 3, 174–182. [Google Scholar] [CrossRef]
- Malkia, A.; Pertusa, M.; Fernández-Ballester, G.; Ferrer-Montiel, A.; Viana, F. Differential role of the menthol-binding residue Y745 in the antagonism of thermally gated TRPM8 channels. Mol. Pain. 2009, 5, 1740–1744. [Google Scholar] [CrossRef] [Green Version]
- Diver, M.M.; Cheng, Y.; Julius, D. Structural insights into TRPM8 inhibition and desensitization. Science 2019, 365, 1434–1440. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 2.0; Schrödinger, LLC: New York, NY, USA, 2015.
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res. 2012, 40, D370–D376. [Google Scholar] [CrossRef]
- Yin, Y.; Le, S.C.; Hsu, A.L.; Borgnia, M.J.; Yang, H.; Lee, S.-Y. Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 2019, 363, eaav9334. [Google Scholar] [CrossRef]
- Chuang, H.; Neuhausser, W.M.; Julius, D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 2004, 43, 859–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, F.J.P.; Knop, G.; Lückhoff, A. The transmembrane segment S6 determines cation versus anion selectivity of TRPM2 and TRPM8. J. Biol. Chem. 2007, 282, 27598–27609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunke, G.M.; Frederick, H.A.; Haught, J.C.; Wos, J.A.; Yelm, K.E. Preparation of Aromatic, Adamantyl, and Highly Branched Aliphatic Compounds, Including Esters and Amides, Useful as Coolants in Oral and Skin Compositions. U.S. Patent US20190330141, 31 October 2019. [Google Scholar]
- Join, B.; Ongouta, J.; Backes, M.; Brodhage, R.; Machinek, A.; Loges, H.; Mundt, S.; Somers, T.; Subkowski, T.; Wittenberg, J.; et al. Use of Physiological Cooling Active Ingredients for Modulation of Menthol Receptor TRPM8, and Compositions Comprising Such Active Ingredients. Patent WO2019043164, 7 March 2019. [Google Scholar]
- Noncovich, A.; Priest, C.; Ung, J.; Patron, A.P.; Servant, G.; Brust, P.; Servant, N.; Faber, N.; Liu, H.; Gonsalves, N.S.; et al. Discovery and development of a novel class of phenoxyacetyl amides as highly potent TRPM8 agonists for use as cooling agents. Bioorganic Med. Chem. Lett. 2017, 27, 3931–3938. [Google Scholar] [CrossRef]
- Cocito Armanino, N.; Bombrun, A.; Chai, A.; Charpentier, J.; Chen, C.; Emter, R.; Mathys, M.; Natsch, A.; Wang, C.; Zhou, L. Preparation of Substituted Azacyles as TRMP8 Modulators. Patent WO2021074281, 22 April 2021. [Google Scholar]
- Cocito Armanino, N.; Charpentier, J.; Chen, C.; Emter, R.; Goeke, A.; Huang, F.; Natsch, A.; Zhou, L.; Zou, Y. Preparation of Octahydrobenzo[c]Isoxazole Derivatives as TRMP8 Agonists Useful as Cooling Sensates. Patent WO2021102896A1, 3 June 2021. [Google Scholar]
- Cocito Armanino, N.; Charpentier, J.; Chen, C.; Emter, R.; Goeke, A.; Huang, F.; Nastch, A.; Zhou, L.; Zou, Y. Organic Compounds. Patent WO2021102896, 3 June 2021. [Google Scholar]
- Wei, E.T. 1-Di(Sec-Butyl)-phosphinoyl-pentane (DAPA-2-5) as a Topical Agent. U.S. Patent 20150111852, 23 April 2015. [Google Scholar]
- Wei, E.T. Di-Isopropyl-phosphinoyl-alkane Compounds as Topical Agents for the Treatment of Sensory Discomfort. Patent WO2015059432, 30 April 2015. [Google Scholar]
- Wei, E.T. Dialkyl-Phosphinoyl-Alkane (DAPA) Compounds and Compositions for Treatment of Lower Gastrointestinal Tract Disorders. U.S. Patent 20170189428, 6 July 2017. [Google Scholar]
- Yang, J.M.; Li, F.; Liu, Q.; Ruedi, M.; Wei, E.T.; Lentsman, M.; Lee, H.S.; Choi, W.; Kim, S.J.; Yoon, K.C. A novel TRPM8 agonist relieves dry eye discomfort. BMC Ophthalmol. 2017, 17, 101. [Google Scholar] [CrossRef] [Green Version]
- Wei, E.T. Method to Reduce Face Mask and Respirator Discomfort. U.S. Patent 20200289533, 17 September 2020. [Google Scholar]
- Terada, Y.; Kitajima, M.; Taguchi, F.; Takayama, H.; Horie, S.; Watanabe, T. Identification of indole alkaloid structural units important for stimulus-selective TRPM8 inhibition: SAR study of naturally occurring Iboga derivatives. J. Nat. Prod. 2014, 77, 1831–1838. [Google Scholar] [CrossRef]
- Bertamino, A.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Soldovieri, M.V.; Iraci, N.; Fernandez Carvajal, A.; de la Torre-Martinez, R.; et al. Tryptamine-based derivatives as Transient Receptor Potential Melastatin Type 8 (TRPM8) channel modulators. J. Med. Chem. 2016, 59, 2179–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Caro, C.; Cristiano, C.; Avagliano, C.; Bertamino, A.; Ostacolo, C.; Campiglia, P.; Gomez-Monterrey, I.; La Rana, G.; Gualillo, O.; Calignano, A.; et al. Characterization of new TRPM8 modulators in pain perception. Int. J. Mol. Sci. 2019, 20, 5544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcas, J.M.; González, A.; Gers-Barlag, K.; González-González, O.; Bech, F.; Demirkhanyan, L.; Zakharian, E.; Belmonte, C.; Gomis, A.; Viana, F. The immunosuppressant macrolide tacrolimus activates cold-sensing TRPM8 channels. J. Neurosci. 2019, 39, 949–969. [Google Scholar] [CrossRef] [PubMed]
- Rimola, V.; Hahnefeld, L.; Zhao, J.; Jiang, C.; Angioni, C.; Schreiber, Y.; Osthues, T.; Pierre, S.; Geisslinger, G.; Ji, R.-R.; et al. Lysophospholipids contribute to oxaliplatin-induced acute peripheral pain. J. Neurosci. 2020, 40, 9519–9532. [Google Scholar] [CrossRef]
- Janssens, A.; Gees, M.; Toth, B.I.; Ghosh, D.; Mulier, M.; Vennekens, R.; Vriens, J.; Talavera, K.; Voets, T. Definition of two agonist types at the mammalian cold-activated channel TRPM8. Elife 2016, 5, e17240. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Li, S.; Zhao, Y.; Liu, Q.; Qiao, Y.; Feng, L.; Li, S. Identification of a natural compound, sesamin, as a novel TRPM8 antagonist with inhibitory effects on prostate adenocarcinoma. Fitoterapia 2020, 145, 104631. [Google Scholar] [CrossRef]
- Sanechika, S.; Shimobori, C.; Ohbuchi, K. Identification of herbal components as TRPA1 agonists and TRPM8 antagonists. J. Nat. Med. 2021, 75, 717–725. [Google Scholar] [CrossRef]
- Tanada, F.; Hirasawa, H.; Mutai, Y.; Kijima, Y.; Kobayashi, J. Preparation of 2-(phenylthiazolyl)benzamide Derivatives as Transient Receptor Potential Cation Channel M8 (TRPM8) Inhibitors. Patent WO2018117166, 28 June 2018. [Google Scholar]
- Hirasawa, H.; Tanada, F.; Mutai, Y.; Fushimi, N.; Kobayashi, J.; Kijima, Y. Preparation of Pyrazole Derivatives as TRPM8 Inhibitors. Patent WO2016208602, 29 December 2016. [Google Scholar]
- Hirasawa, H.; Tanada, F.; Mutai, Y.; Fushimi, N.; Kobayashi, J.; Kijima, Y. Pharmaceutical Composition Containing Pyrazole Derivatives as TRPM8 Inhibitors. Patent JP2018100269, 28 June 2018. [Google Scholar]
- Hirasawa, H.; Tanada, F.; Mutai, Y.; Fushimi, N.; Kobayashi, J.; Kijima, Y. Method for the Preparation of Pyrazole Derivatives. Patent JP2018108988, 7 December 2018. [Google Scholar]
- Nakanishi, O.; Fujimori, Y.; Aizawa, N.; Hayashi, T.; Matsuzawa, A.; Kobayashi, J.I.; Hirasawa, H.; Mutai, Y.; Tanada, F.; Igawa, Y. KPR-5714, a novel transient receptor potential melastatin 8 antagonist, improves overactive bladder via inhibition of bladder afferent hyperactivity in rats. J. Pharmacol. Exp. Ther. 2020, 373, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, N.; Fujimori, Y.; Nakanishi, O.; Hayashi, T.; Goi, Y.; Kobayashi, J.; Fujita, T. Efficacy of the combination of KPR-5714, a novel transient receptor potential melastatin 8 (TRPM8) antagonist, and β3-adrenoceptor agonist or anticholinergic agent on bladder dysfunction in rats with bladder overactivity. Eur. J. Pharmacol. 2021, 899, 173995. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, H.; Kawamura, N.; Kobayashi, J.; Ozawa, T. Preparation of α-Substituted Glycinamide Derivatives and Their Salts as TRPM8 Blocking Agents, and Pharmaceutical Compositions Containing Them. Patent WO2015108136, 23 July 2015. [Google Scholar]
- Hirasawa, H.; Kawamura, N.; Kobayashi, J. TRPMB Inhibitors Containing α-Substituted Glycine Amides. Patent WO2014181788, 13 November 2014. [Google Scholar]
- Kobayashi, J.; Hirasawa, H.; Fujimori, Y.; Nakanishi, O.; Kamada, N.; Ikeda, T.; Yamamoto, A.; Kanbe, H. Identification of N-acyl-N-indanyl-α-phenylglycinamides as selective TRPM8 antagonists designed to mitigate the risk of adverse effects. Bioorg. Med. Chem. 2021, 30, 115903. [Google Scholar] [CrossRef]
- Tsuzuki, Y.; Sawamoto, D.; Sakamoto, T.; Kato, T.; Niwa, Y.; Awai, N. Preparation of Sulfonamide Compounds Having TRPM8 Antagonistic Activity. Patent WO2012124825, 20 September 2012. [Google Scholar]
- Kato, T.; Sakamoto, T.; Kubo, A.; Sawamoto, D. Sulfonamides Compounds. Patent WO2014042238, 20 March 2014. [Google Scholar]
- Palumbo, J.M. Compositions for Treating or Preventing Vasomotor Symptoms. Patent WO2017217351, 21 December 2017. [Google Scholar]
- Komasaka, T. Preparation of Crystal Forms of 4-[[(4-cyclopropylisoquinolin-3-yl)[4-(trifluoromethoxy)benzyl]amino]sulfonyl]benzoic Acid. Patent JP2019116445, 18 July 2019. [Google Scholar]
- Kato, T.; Sakamoto, T.; Niwa, Y.; Sawamoto, D.; Ohtani, N.; Kanbe, M. Aromatic Carboxylic acid Amide Compounds. Patent WO2016039448, 17 March 2016. [Google Scholar]
- Kato, T.; Sakamoto, T.; Niwa, Y.; Sawamoto, D.; Otani, N.; Kanbe, M. Preparation of Aromatic Carboxylic Acid Amides Having TRPM8 Blocking Effect. Patent JP2017214290, 7 December 2017. [Google Scholar]
- Horne, D.B.; Biswas, K.; Brown, J.; Bartberger, M.D.; Clarine, J.; Davis, C.D.; Gore, V.K.; Harried, S.; Horner, M.; Kaller, M.R.; et al. Discovery of TRPM8 antagonist (S)-6-(((3-fluoro-4-(trifluoromethoxy)phenyl)(3-fluoropyridin-2-yl)methyl)carbamoyl)nicotinic acid (AMG 333), a clinical candidate for the treatment of migraine. J. Med. Chem. 2018, 61, 8186–8201. [Google Scholar] [CrossRef]
- Aizawa, N.; Ohshiro, H.; Watanabe, S.; Kume, H.; Homma, Y.; Igawa, Y. RQ-00434739, a novel TRPM8 antagonist, inhibits prostaglandin E2-induced hyperactivity of the primary bladder afferent nerves in rats. Life Sci. 2019, 218, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Journigan, V.B.; Alarcon-Alarcon, D.; Feng, Z.; Wang, Y.; Liang, T.; Dawley, D.C.; Amin, A.R.M.R.; Montano, C.; Van Horn, W.D.; Xie, X.-Q.; et al. Structural and in vitro functional characterization of a menthyl TRPM8 antagonist indicates species-dependent regulation. ACS Med. Chem. Lett. 2021, 12, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Bertamino, A.; Iraci, N.; Ostacolo, C.; Ambrosino, P.; Musella, S.; Di Sarno, V.; Ciaglia, T.; Pepe, G.; Sala, M.; Soldovieri, M.V.; et al. Identification of a potent tryptophan-based TRPM8 antagonist with in vivo analgesic activity. J. Med. Chem. 2018, 61, 6140–6152. [Google Scholar] [CrossRef] [PubMed]
- Bertamino, A.; Ostacolo, C.; Medina, A.; Di Sarno, V.; Lauro, G.; Ciaglia, T.; Vestuto, V.; Pepe, G.; Basilicata, M.G.; Musella, S.; et al. Exploration of TRPM8 binding sites by β-carboline-based antagonists and their in vtro characterization and in vivo analgesic activities. J. Med. Chem. 2020, 63, 9672–9694. [Google Scholar] [CrossRef]
- Perez-Faginas, P.; Teresa Aranda, M.; de la Torre-Martinez, R.; Quirce, S.; Fernandez-Carvajal, A.; Ferrer-Montiel, A.; Gonzalez-Muniz, R. New transient receptor potential TRPV1, TRPM8 and TRPA1 channel antagonists from a single linear β,γ-diamino ester scaffold. RSC Adv. 2016, 6, 6868–6877. [Google Scholar] [CrossRef]
- De la Torre-Martínez, R.; Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; Bonache, M.A.; Llabres-Campaner, P.J.; Balsera, B.; de Perez, V.M.J.; Gonzalez-Muniz, R. Synthesis, high-throughput screening and pharmacological characterization of β-lactam derivatives as TRPM8 antagonists. Sci. Rep. 2017, 7, 10766. [Google Scholar] [CrossRef] [Green Version]
- Bonache, M.Á.; Martín-Escura, C.; de la Torre Martínez, R.; Medina, A.; González-Rodríguez, S.; Francesch, A.; Cuevas, C.; Roa, A.M.; Fernández-Ballester, G.; Ferrer-Montiel, A.; et al. Highly functionalized β-lactams and 2-ketopiperazines as TRPM8 antagonists with antiallodynic activity. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Bonache, M.A.; Llabres, P.J.; Martin-Escura, C.; De la Torre-Martinez, R.; Medina-Peris, A.; Butron, L.; Gomez-Monterrey, I.; Roa, A.M.; Fernandez-Ballester, G.; Ferrer-Montiel, A.; et al. Phenylalanine-derived β-lactam TRPM8 modulators. configuration effect on the antagonist activity. Int. J. Mol. Sci. 2021, 22, 2370. [Google Scholar] [CrossRef] [PubMed]
- Zandi, N.; Zaniani, N.R.; Moghimi, A.; Roohbakhsh, A. Protective effects of M8-B, a TRPM8 antagonist, on febrile- and pentylenetetrazol-induced seizures. Acta Neurobiol. Exp. 2019, 79, 86–91. [Google Scholar]
- Andersen, H.H.; Gazerani, P.; Arendt-Nielsen, L. High-concentration L-menthol exhibits counter-irritancy to neurogenic inflammation, thermal and mechanical hyperalgesia caused by trans-cinnamaldehyde. J. Pain 2016, 17, 919–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topp, R.; Brosky, J.A.J.; Pieschel, D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J. Geriatr. Phys. Ther. 2013, 36, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Cortellini, A.; Verna, L.; Cannita, K.; Napoleoni, L.; Parisi, A.; Ficorella, C.; Porzio, G. Topical menthol for treatment of chemotherapy-induced peripheral neuropathy. Indian J. Palliat. Care 2017, 23, 350–352. [Google Scholar] [CrossRef]
- Sundstrup, E.; Jakobsen, M.D.; Jay, K.; Brandt, M.; Andersen, L.L.; Colado, J.C.; Wang, Y. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: Triple-blind, randomized placebo-controlled trial. Rehabil. Res. Pract. 2014, 2014, 310913. [Google Scholar] [CrossRef] [Green Version]
- St. Cyr, A.; Chen, A.; Bradley, K.C.; Yuan, H.; Silberstein, S.D.; Young, W.B. Efficacy and tolerability of STOPAIN for a migraine attack. Front. Neurol. 2015, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Kayama, Y.; Shibata, M.; Takizawa, T.; Shimizu, T.; Ebine, T.; Toriumi, H.; Suzuki, N.; Ibata, K.; Yuzaki, M. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: Relevance to migraine pathophysiology. Cephalalgia 2018, 38, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yang, T.; Wang, P.; Ma, S.; Zhu, Z.; Pu, Y.; Li, L.; Zhao, Y.; Xiong, S.; Liu, D.Z. Activation of cold-sensing transient receptor potential melastatin subtype 8 antagonizes vasoconstriction and hypertension through attenuating RhoA/Rho kinase pathway. Hypertension 2014, 63, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Huet, F.; Misery, L.; Huet, F.; Santerre, A.; Neufang, G.; Batardiere, A.; Hornez, N.; Nedelec, A.S.; Le Caёr, F.; et al. Real-life study of anti-itching effects of a cream containing menthoxypropanediol, a TRPM8 agonist, in atopic dermatitis patients. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e67–e69. [Google Scholar] [CrossRef]
- Jung, M.J.; Kim, J.C.; Wei, E.T.; Selescu, T.; Chung, B.Y.; Park, C.W.; Kim, H.O. A randomized, vehicle-controlled clinical trial of a synthetic TRPM8 agonist (Cryosim-1) gel for itch. J. Am. Acad. Dermatol. 2021, 84, 869–871. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, J.; Yoon, K.C.; Yang, J.M.; Wei, E.T.; Kim, S.J. Topical TRPM8 agonist for relieving neuropathic ocular pain in patients with dry eye: A pilot study. J. Clin. Med. 2021, 10, 250. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Patnaik, A.; Papadopoulos, K.; Mays, T.; Stephan, T.; Humble, D.J.; Frohlich, M.W.; Sims, R.B. Preliminary results from a Phase 1 study of D-3263 HCl, a TRPM8 calcium channel agonist, in patients with advanced cancer. Eur. J. Cancer Suppl. 2010, 8, 119. [Google Scholar] [CrossRef]
- Rovner, S.L. Better Than Mint Medicinal chemistry methods lead to new cooling compounds that are more potent and last longer than menthol. Chem. Eng. News 2005, 85, 95–98. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [Green Version]
- Andrews, M.D.; Af Forselles, K.; Beaumont, K.; Galan, S.R.G.; Glossop, P.A.; Grenie, M.; Jessiman, A.; Kenyon, A.S.; Lunn, G.; Maw, G.; et al. Discovery of a selective TRPM8 antagonist with clinical efficacy in cold-related pain. ACS Med. Chem. Lett. 2015, 6, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Meye, A.; Fuessel, S.; Koch, R.; Unversucht, S.; Wirth, M.P. Genetic Markers for Diagnosis and Differentiation of Prostate Cancer. Patent DE102006032394, 7 July 2007. [Google Scholar]
- Nomura, D.; Tsukimoto, M.; Abe, R. Involvement of TRPM8 channel in radiation-induced DNA damage repair mechanism contributing to radioresistance of B16 melanoma. Biol. Pharm. Bull. 2021, 44, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Kupari, J.; Usoskin, D.; Parisien, M.; Lou, D.; Hu, Y.; Fatt, M.; Loennerberg, P.; Spaangberg, M.; Eriksson, B.; Barkas, N.; et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun. 2021, 12, 1510. [Google Scholar] [CrossRef]
- Soeda, M.; Ohka, S.; Nishizawa, D.; Hasegawa, J.; Nakayama, K.; Ebata, Y.; Ikeda, K.; Soeda, M.; Fukuda, K.-I.; Ichinohe, T. Cold pain sensitivity is associated with single-nucleotide polymorphisms of PAR2/F2RL1 and TRPM8. Mol. Pain 2021, 17, 17448069211002008. [Google Scholar] [CrossRef]
- Lelis Carvalho, A.; Treyball, A.; Brooks, D.J.; Costa, S.; Neilson, R.J.; Reagan, M.R.; Bouxsein, M.L.; Motyl, K.J. TRPM8 modulates temperature regulation in a sex-dependent manner without affecting cold-induced bone loss. PLoS ONE 2021, 16, e0231060. [Google Scholar] [CrossRef]
- Kanda, H.; Ling, J.; Chang, Y.-T.; Erol, F.; Viatchenko-Karpinski, V.; Yamada, A.; Noguchi, K.; Gu, J.G. Kv4.3 channel dysfunction contributes to trigeminal neuropathic pain manifested with orofacial cold hypersensitivity in rats. J. Neurosci. 2021, 41, 2091–2105. [Google Scholar] [CrossRef]
- Iftinca, M.; Basso, L.; Flynn, R.; Kwok, C.; Roland, C.; Hassan, A.; Defaye, M.; Ramachandran, R.; Trang, T.; Altier, C. Chronic morphine regulates TRPM8 channels via MOR-PKCβ signaling. Mol. Brain 2020, 13, 61. [Google Scholar] [CrossRef] [Green Version]
- Rivera, B.; Campos, M.; Madrid, R.; Pertusa, M.; Orio, P. Negative modulation of TRPM8 channel function by protein kinase C in trigeminal cold thermoreceptor neurons. Int. J. Mol. Sci. 2020, 21, 4420. [Google Scholar] [CrossRef] [PubMed]
- Acharya, T.K.; Kumar, S.; Tiwari, N.; Ghosh, A.; Tiwari, A.; Pal, S.; Majhi, R.K.; Kumar, A.; Das, R.; Singh, A.; et al. TRPM8 channel inhibitor-encapsulated hydrogel as a tunable surface for bone tissue engineering. Sci. Rep. 2021, 11, 3730. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo, C.; Martín-Martínez, M.; Gómez-Monterrey, I.; González-Muñiz, R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. Int. J. Mol. Sci. 2021, 22, 8502. https://doi.org/10.3390/ijms22168502
Izquierdo C, Martín-Martínez M, Gómez-Monterrey I, González-Muñiz R. TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. International Journal of Molecular Sciences. 2021; 22(16):8502. https://doi.org/10.3390/ijms22168502
Chicago/Turabian StyleIzquierdo, Carolina, Mercedes Martín-Martínez, Isabel Gómez-Monterrey, and Rosario González-Muñiz. 2021. "TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation" International Journal of Molecular Sciences 22, no. 16: 8502. https://doi.org/10.3390/ijms22168502
APA StyleIzquierdo, C., Martín-Martínez, M., Gómez-Monterrey, I., & González-Muñiz, R. (2021). TRPM8 Channels: Advances in Structural Studies and Pharmacological Modulation. International Journal of Molecular Sciences, 22(16), 8502. https://doi.org/10.3390/ijms22168502